Global Porokeratosis Treatment Market Insights Forecasts to 2035
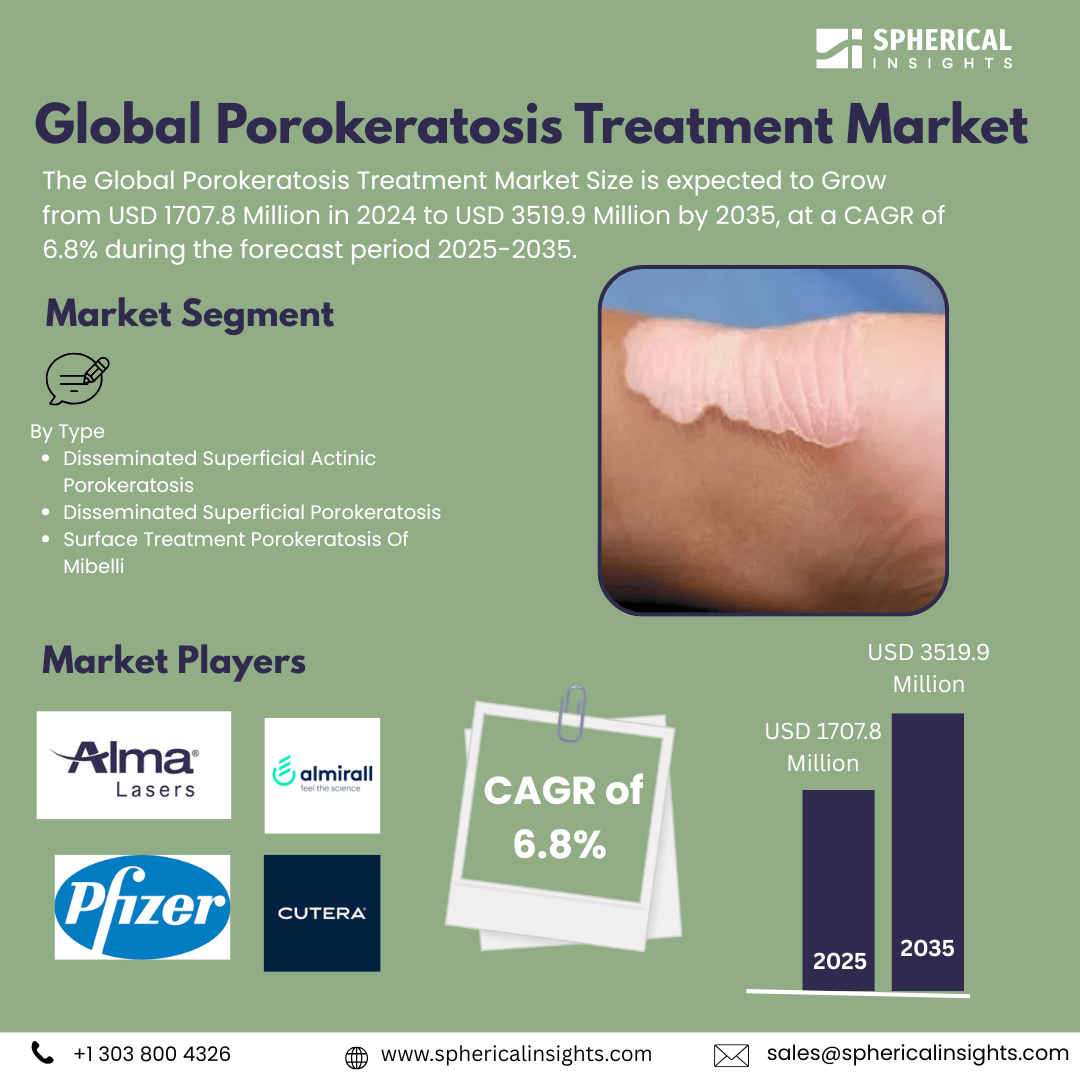
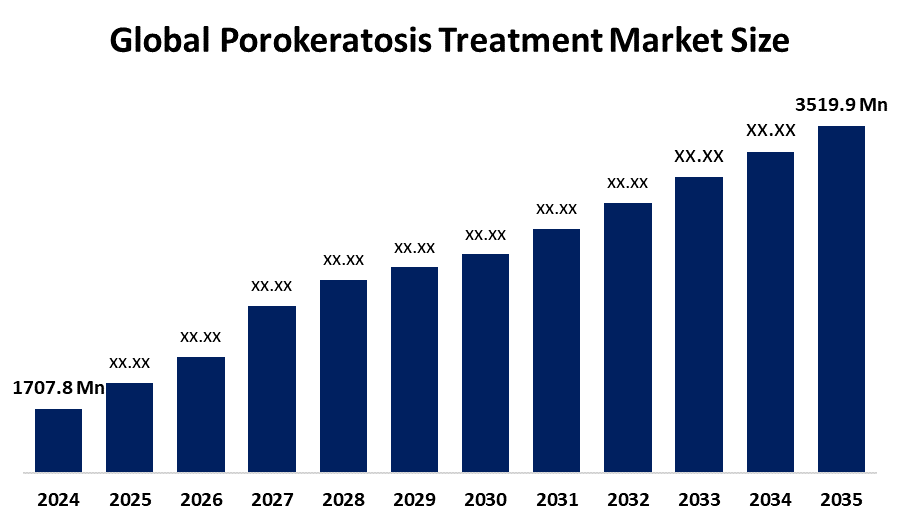
- The Global Porokeratosis Treatment Market Size Was Estimated at USD 1707.8 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 6.8% from 2025 to 2035
- The Worldwide Porokeratosis Treatment Market Size is Expected to Reach USD 3519.9 Million by 2035
- Asia Pacific is expected to grow the fastest during the forecast period.
Porokeratosis Treatment Market
The Porokeratosis Treatment Market Size focuses on therapies for a rare group of skin disorders characterized by abnormal keratinization, leading to lesions with a distinctive ridge-like border. Treatment options include topical medications (such as 5-fluorouracil, retinoids), cryotherapy, laser therapy, and emerging gene-based therapies. Market growth is driven by rising awareness, increasing prevalence of rare dermatological conditions, and advancements in dermatology. Governments and health organizations are supporting rare disease research through funding, faster drug approvals, and orphan drug designation initiatives. In regions like the U.S. and EU, regulatory frameworks encourage innovation in rare disease treatment, including porokeratosis. Additionally, collaborations between pharmaceutical companies and research institutions are accelerating the development of targeted treatments. Despite being a niche market, growing patient demand for effective and cosmetic-friendly therapies boosts interest in new product development. Overall, the market is poised for moderate growth, fueled by innovation, supportive policies, and a greater focus on personalized dermatological care.
Attractive Opportunities in the Porokeratosis Treatment Market
- Pharmaceutical companies can tap into the underserved niche by developing safer, more effective, and cosmetically acceptable treatments, such as gene-based therapies and targeted molecular therapies, which promise longer-lasting results.
- Teledermatology and digital health platforms enhance diagnosis and access to care, especially in remote or underserved areas, opening new channels for patient reach and treatment delivery.
- Growing public awareness, patient advocacy, and global expansion of orphan drug programs create a favorable regulatory environment for innovation, encouraging pharma companies to invest in porokeratosis treatment development.
Global Porokeratosis Treatment Market Dynamics
DRIVER: Expanding availability of advanced therapies
Increasing awareness of rare skin disorders and advancements in dermatological diagnostics contribute to early detection and demand for treatment. Rising investments in research and development, along with supportive government initiatives such as orphan drug designations and funding for rare diseases, further stimulate innovation. The expanding availability of advanced therapies, including laser treatments and topical agents, also supports market growth. Additionally, growing healthcare access in developing regions and the increasing demand for personalized and minimally invasive treatments are expected to propel the market forward in the coming years.
RESTRAINT: High treatment costs
Despite its growth potential, the porokeratosis treatment market faces several restraining factors. Limited awareness among general practitioners and delayed diagnosis can hinder early treatment. The rarity of the condition results in a smaller patient population, reducing commercial incentives for pharmaceutical companies. High treatment costs, especially for advanced therapies, and limited insurance coverage in some regions further restrict patient access. Additionally, the lack of standardized treatment protocols and the variable effectiveness of existing therapies can lead to inconsistent outcomes. Regulatory challenges and the lengthy approval process for new treatments, especially for rare diseases, also pose significant barriers to market expansion.
OPPORTUNITY: Pharmaceutical companies have the opportunity to tap into this underserved niche by developing safer
Emerging therapies, such as gene-based treatments and targeted molecular therapies, offer promising avenues for more effective and long-lasting results. Increasing investment in rare disease research and the expansion of orphan drug programs globally create a favorable regulatory environment for innovation. Teledermatology and digital health tools also enhance diagnosis and access to care, especially in remote areas. Moreover, growing public awareness and patient advocacy for rare skin conditions are pushing demand for specialized treatments. Pharmaceutical companies have the opportunity to tap into this underserved niche by developing safer, more efficient, and cosmetically acceptable solutions, creating potential for both medical breakthroughs and market expansion over the coming years.
CHALLENGES: Lack of standardized treatment guidelines leads to inconsistent care
A lack of standardized treatment guidelines leads to inconsistent care. High development costs and uncertain return on investment deter pharmaceutical companies. Additionally, patient awareness remains low, delaying diagnosis and treatment. Access to advanced therapies is limited in low-income regions, and regulatory hurdles for rare disease treatments further complicate market entry and growth.
Global Porokeratosis Treatment Market Ecosystem Analysis
The global porokeratosis treatment market ecosystem includes pharmaceutical companies, healthcare providers, regulatory bodies, research institutions, and patient advocacy groups. Key players develop topical, laser, and cryotherapy treatments, supported by hospitals and clinics delivering care. Regulatory agencies ensure safety and efficacy, while research drives innovation. Market growth is fueled by rising awareness, technological advancements, and government initiatives. Challenges include high costs, limited patient numbers, and regulatory hurdles. Regions like North America lead, with Europe and Asia-Pacific showing strong growth potential, driven by expanding healthcare access and increasing diagnosis rates.
Based on the type, the disseminated superficial actinic porokeratosis segment led the market with the largest revenue share over the forecast period
The disseminated superficial actinic porokeratosis segment dominance is primarily due to DSAP’s higher prevalence compared to other porokeratosis types, especially in sun-exposed populations. The chronic nature of DSAP, characterized by multiple lesions, creates sustained demand for effective treatment options. Additionally, increasing awareness among dermatologists and patients about DSAP’s potential to progress to skin cancer drives early diagnosis and treatment. Advances in topical therapies, laser treatments, and non-invasive procedures specifically targeting DSAP further fuel market growth in this segment.
North America is anticipated to hold the largest market share of the porokeratosis treatment market during the forecast period
North America is anticipated to hold the largest market share in the porokeratosis treatment market during the forecast period. This is attributed to the region’s advanced healthcare infrastructure, high awareness of rare skin disorders, and strong presence of leading pharmaceutical and biotechnology companies. Additionally, robust government support through funding and favorable regulatory policies for orphan drugs accelerates research and development. The availability of advanced diagnostic tools and treatment options, combined with a growing patient base, further strengthens North America’s dominant position in the market.
Asia Pacific is expected to grow at the fastest CAGR in the porokeratosis treatment market during the forecast period
Asia Pacific is expected to grow at the fastest CAGR in the porokeratosis treatment market during the forecast period. This rapid growth is driven by increasing healthcare infrastructure development, rising awareness about rare skin diseases, and expanding access to advanced dermatological treatments. Growing investments in healthcare, improving reimbursement policies, and a large patient population also contribute to market expansion. Additionally, rising disposable incomes and growing urbanization are enabling more patients to seek specialized care, making Asia Pacific a key emerging market for porokeratosis treatments.
Recent Development
- In January 2022, Pfizer Inc. received FDA approval for CIBINQO (abrocitinib), an oral Janus kinase 1 (JAK1) inhibitor for treating adults with moderate-to-severe atopic dermatitis. While primarily for atopic dermatitis, its approval reflects the growing focus on dermatological treatments.
Key Market Players
KEY PLAYERS IN THE POROKERATOSIS TREATMENT MARKET INCLUDE
- Pfizer Inc.
- Alma Lasers
- Almirall, S.A.
- Cutera, Inc.
- Lumenis Ltd.
- Sun Pharmaceutical Industries Ltd.
- Allergan (AbbVie Inc.)
- Coherent, Inc.
- Hugel, Inc.
- Medi-Globe Corporation
- Others
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the porokeratosis treatment market based on the below-mentioned segments:
Global Porokeratosis Treatment Market, By Type
- Disseminated Superficial Actinic Porokeratosis
- Disseminated Superficial Porokeratosis
- Surface Treatment Porokeratosis Of Mibelli
Global Porokeratosis Treatment Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
FAQs
Q: What are the main drivers of growth in the Porokeratosis Treatment Market?
A: Key drivers include rising awareness of rare skin disorders, advancements in dermatological diagnostics and therapies, supportive government initiatives, and increasing demand for personalized and minimally invasive treatments.
Q: What are the major challenges limiting the adoption of Porokeratosis treatments?
A: Challenges include high treatment costs, limited patient population, lack of standardized treatment protocols, regulatory hurdles, and limited awareness leading to delayed diagnosis.
Q: What are the attractive opportunities in the Porokeratosis Treatment Market?
A: Opportunities lie in developing safer and more effective treatments such as gene-based and molecular therapies, expanding teledermatology services, increasing public awareness, and leveraging orphan drug programs.
Q: Which segment leads the market by type in Porokeratosis Treatment?
A: The disseminated superficial actinic porokeratosis (DSAP) segment leads the market due to its higher prevalence and demand for effective treatment.
Q: Who are the key players operating in the Global Porokeratosis Treatment Market?
A: Major players include Pfizer Inc., Alma Lasers, Almirall, S.A., Cutera, Inc., Lumenis Ltd., Sun Pharmaceutical Industries Ltd., Allergan (AbbVie Inc.), Coherent, Inc., Hugel, Inc., and Medi-Globe Corporation.
Q: What recent developments have occurred in the Porokeratosis Treatment Market?
A: In January 2022, Pfizer Inc. received FDA approval for CIBINQO (abrocitinib), an oral JAK1 inhibitor for moderate-to-severe atopic dermatitis, signaling growth in dermatological treatments.
Q: How does the regulatory environment impact the Porokeratosis Treatment Market?
A: Regulatory support through orphan drug designation, faster drug approvals, and government funding for rare diseases fosters innovation and market growth, especially in regions like the U.S. and Europe.
Q: What are the emerging trends in the Porokeratosis Treatment Market?
A: Emerging trends include gene-based therapies, targeted molecular treatments, teledermatology platforms, and personalized dermatological care.
Q: How is the Porokeratosis Treatment Market segmented regionally?
A: The market is segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, with further country-level breakdowns such as the U.S., Germany, China, Brazil, and UAE.
Q: What is the long-term outlook for the Porokeratosis Treatment Market?
A: The market is poised for moderate growth through 2035, driven by innovation, increasing healthcare access, and rising patient demand for effective, cosmetically acceptable treatments.