Pompe Disease Treatment Market: Understanding and Treatment Algorithm:
Pompe Disease is a rare genetic disorder caused by a deficiency of the enzyme acid alpha-glucosidase (GAA), which breaks down glycogen in cells. Without this enzyme, glycogen accumulates in muscles and other tissues, leading to progressive muscle weakness, respiratory issues, and heart problems, especially in infantile-onset cases.
Pompe Disease Diagnosis:
Pompe Disease is diagnosed through a combination of clinical evaluation and laboratory tests. Key methods include blood tests to measure GAA enzyme activity, genetic testing to confirm mutations in the GAA gene, and muscle biopsies in some cases. Newborn screening programs also help detect Pompe Disease early for timely intervention.
Pompe Disease Treatment:
Treatment for Pompe Disease primarily involves enzyme replacement therapy (ERT) using medications like alglucosidase alfa to restore GAA enzyme levels and reduce glycogen buildup. Supportive care includes respiratory support, physical therapy, and nutritional management. Emerging treatments, such as gene therapies, aim to offer long-term correction of the underlying enzyme deficiency.
Pompe Disease Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Pompe Disease, Gender-specific Diagnosed Incidence of Pompe Disease, Type specific Diagnosed Incidence of Pompe Disease, Age specific Diagnosed Incidence of Pompe Disease, Diagnosed Incident Population based on Primary Site of Pompe Disease, and Diagnosed Incident Population based on Histologic Classification of Pompe Disease Tumour in the global market covering North America, Europe, Asia Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of Pompe disease epidemiology in major markets worldwide.
Country-Wise Pompe Disease Multiforme Epidemiology
- The epidemiology segment provides Pompe Disease prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia Pacific (including Japan), Latin America, the Middle East, and Africa.
Pompe Disease: Recent Developments:
- In August 2021, Sanofi announced that the U.S. FDA had approved Nexviazyme (avalglucosidase alfa ngpt) for the treatment of late onset Pompe disease in patients aged one year and older. The approval was based on clinical trial results demonstrating improved respiratory function and mobility, marking a major advancement in enzyme replacement therapy for this condition.
Pompe Disease Marketed Drugs:
Myozyme (alglucosidase alfa) is a recombinant human GAA enzyme used for enzyme replacement therapy in infantile onset Pompe Disease. It helps break down glycogen accumulation in cells by restoring enzyme function. Myozyme is FDA approved and administered intravenously to improve survival and motor function in affected patients.
Lumizyme (alglucosidase alfa) is the commercial version of Myozyme for late onset Pompe Disease. It is approved for use in patients older than 8 years in the U.S. and provides long term enzyme replacement to reduce muscle weakness and respiratory issues caused by glycogen buildup.
- Nexviazyme: Sanofi Genzyme
Nexviazyme (avalglucosidase alfa) is a next generation enzyme replacement therapy for late onset Pompe Disease. It is designed for enhanced targeting and uptake in muscle tissues via optimized mannose 6 phosphate receptor binding. Approved by the FDA, Nexviazyme offers improved efficacy in maintaining respiratory and motor function.
Pompe Disease: Emerging Therapies:
- AT845: It is an AAV8 based gene therapy in clinical trials for late onset Pompe Disease. It delivers a functional copy of the GAA gene to muscle cells, aiming to restore enzyme activity and reduce glycogen accumulation. AT845 offers a potential one time treatment alternative to lifelong enzyme replacement therapy.
- SPK-3006: It is an investigational gene therapy that uses an AAV vector to deliver the GAA gene in Pompe patients. It is designed to provide sustained expression of the deficient enzyme, improving muscle function and reducing disease burden. The therapy is currently undergoing early phase clinical evaluation.
- AVR RD 03: It is an ex vivo lentiviral gene therapy in preclinical development for Pompe Disease. It involves modifying a patient’s hematopoietic stem cells to express the GAA enzyme, to achieve durable enzyme production and eliminate the need for chronic ERT.
- ACTUS 101: It is an investigational AAV based gene therapy aimed at delivering the GAA gene directly to muscle tissues. It is designed to provide long term enzyme expression from a single dose, potentially replacing the need for frequent enzyme replacement therapy. The therapy is in early clinical development stages.
Pompe Disease Market Outlook
- The Pompe Disease market includes drugs, diagnostics, and gene therapies targeting Pompe Disease, a rare lysosomal storage disorder caused by GAA enzyme deficiency. Treatments focus on enzyme replacement and emerging genetic interventions, addressing both infantile and late onset forms to slow disease progression and improve survival.
- Key drivers include growing awareness of rare diseases, availability of newborn screening, improved diagnostic tools, and the increasing use of enzyme replacement therapies (ERT). Regulatory incentives for orphan drugs and advancements in genetic research also support the rapid development and commercialization of Pompe specific treatments.
- Opportunities lie in gene therapy innovation, personalized treatment approaches, and expanding clinical trial activity. Emerging markets with improved healthcare infrastructure and screening programs present growth potential. Partnerships between biotech firms and academic institutions are fueling drug development pipelines targeting both early and late stage Pompe disease.
- Governments support this market through orphan drug incentives, rare disease research funding, and implementation of newborn screening programs. Regulatory agencies offer fast track designations and financial backing to accelerate Pompe disease therapy development and ensure broader access to life saving treatments.
- High cost of treatment and limited patient population constrain widespread access.
- The market is expected to grow due to increased adoption of gene therapy and improved diagnosis through newborn screening.
Pompe Disease Market Segmentation
By Type:
- Classic Infantile Form
- NonClassic Infantile Form
- Late Onset Form
- Others
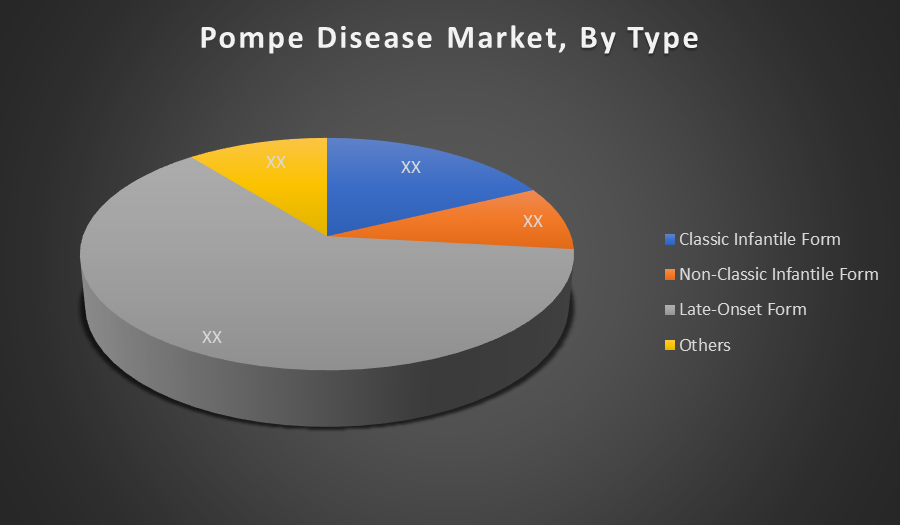
Late-Onset Form holds the largest market share because it affects a broader age group, especially adults, leading to a higher patient population under treatment. LOPD progresses slowly but requires lifelong enzyme replacement therapy, which drives continuous demand. The chronic management approach makes this segment more commercially significant than infantile forms.
By Diagnosis:
- Blood Test
- Genetic Test
- Prenatal Test
- Others
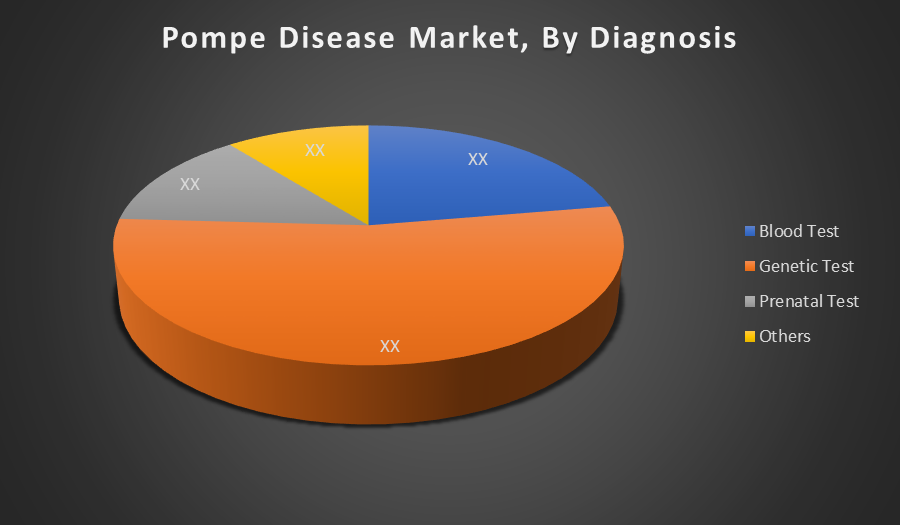
Genetic Test dominates the diagnostic segment because it provides the most accurate and definitive method for identifying Pompe Disease by detecting mutations in the GAA gene. It supports early intervention, newborn screening, and even prenatal diagnosis. As testing technology becomes more affordable and widespread, its adoption continues to rise, securing its leading market position.
Regional Segment Analysis of the Pompe Disease Market
North America dominated the Pompe Disease drug market due to robust healthcare infrastructure, early access to enzyme replacement therapies (ERTs), and strong regulatory support, including orphan drug incentives and rapid FDA approvals. Significant R&D investments by major biotech firms and active patient advocacy further cemented the region’s market dominance.
The Asia Pacific region showed the fastest growth in the Pompe Disease market, propelled by increasing healthcare spending, expanding genetic disease awareness, and improving regulatory frameworks in countries like China, India, Japan, and South Korea. Partnerships between global pharma and local institutions enriched clinical trial activity and access to treatments. Enhanced newborn screening adoption and rising diagnosis rates accelerated market expansion.
Pompe Disease Market Key Companies
- Sanofi
- Amicus Therapeutics
- Audentes Therapeutics
- Spark Therapeutics
- Genzyme Corporation
- Valerion Therapeutics
- AVROBIO
- Biomarin Pharmaceutical
- Pfizer Inc.
- Novartis AG
- Takeda Pharmaceutical
- Asklepios BioPharmaceutical
- Actus Therapeutics
- Astellas Pharma
- EpiVax Inc.
- Others
Pompe Disease Therapeutics Market Report Scope
- The Pompe Disease therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Pompe Disease’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Pompe Disease therapies is provided, including an evaluation of new treatments expected to influence the current Pompe Disease treatment market landscape.
- The report includes a detailed review of the Pompe Disease therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient Based Pompe Disease Market Forecasting report offers valuable insights into trends shaping the global Pompe Disease market, helping to develop effective business strategies.
Pompe Disease Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Pompe Disease Therapeutic Approaches in Pompe Disease
- Review Of Drugs in Development for Pompe Disease
- Market, Growth, and Trends in Pompe Disease
- Market Opportunities in Pompe Disease Treatment
- Effects Of Future Therapies on Pompe Disease Treatment.
Pompe Disease Treatment Market Report Key Strengths
- 15 Years Pompe Disease Market Forecast
- Global Coverage
- Pompe Disease Epidemiology Segmentation
- Key Cross Competition
Pompe Disease Treatment Market Report Assessment
- Present Practices in the Pompe Disease Treatment Market
- Review of Investigational Pompe Disease Drugs
- Attractiveness of the Pompe Disease Drug Market
- Pompe Disease Market Drivers
- Pompe Disease Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Pompe disease market based on the below mentioned segments:
Global Pompe Disease Market, By Type
- Classic Infantile Form
- Non Classic Infantile Form
- Late Onset Form
- Others
Global Pompe Disease Market, By Diagnosis
- Blood Test
- Genetic Test
- Prenatal Test
- Others
Global Pompe Disease Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa