mRNA Quality Monitoring Market Summary
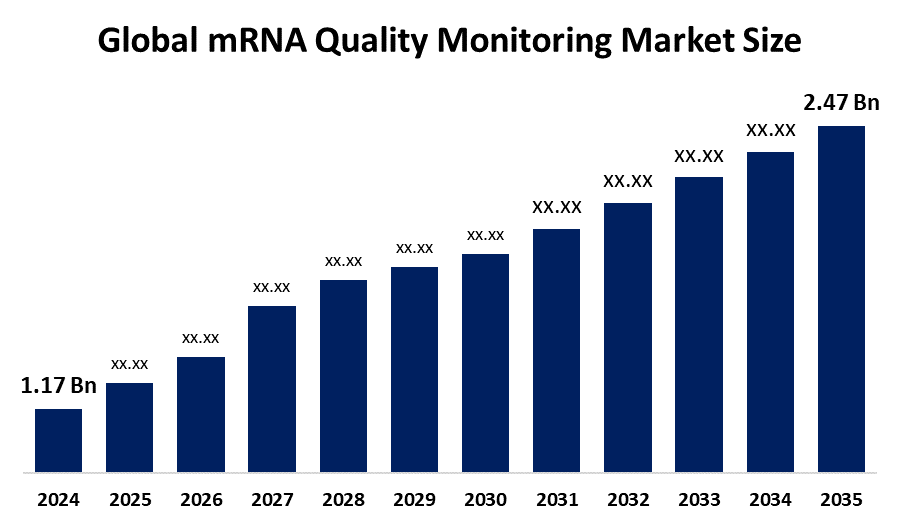
The Global mRNA Quality Monitoring Market Size Was Estimated at USD 1.17 Billion in 2024 and is Projected to Reach USD 2.47 Billion by 2035, Growing at a CAGR of 7.03% from 2025 to 2035. Growing mRNA-based treatments and vaccines, increased demand for quality control in biomanufacturing, improvements in analytical tool technology, heightened regulatory attention to product safety, and rising investments in biotechnology research are the main factors propelling the mRNA quality monitoring market.
Key Regional and Segment-Wise Insights
- In 2024, the North America mRNA quality monitoring market size held the largest revenue share of 35.13% and dominated the global market.
- In 2024, the kits/assays & reagents segment held the highest revenue share of 51.82% and dominated the global market size by product & service.
- With the biggest revenue share of 32.16% in 2024, the electrophoresis segment led the worldwide mRNA quality monitoring market size by technology.
Global Market Forecast and Revenue Outlook
- 2024 Market Size: USD 1.17 Billion
- 2035 Projected Market Size: USD 2.47 Billion
- CAGR (2025-2035): 7.03%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
The mRNA quality monitoring market size includes all tools and technologies, and services, which assess the purity, integrity, and potency of messenger RNA (mRNA) molecules throughout their entire research and development, and production process. The development of safe and effective mRNA-based treatments and vaccines requires the production of high-quality mRNA. The market growth mainly results from the rapid advancement of mRNA technology, which gained momentum after COVID-19 vaccines demonstrated their effectiveness. This led to increased funding for mRNA production and research. The increasing adoption of mRNA platforms for infectious disease treatment, cancer immunotherapy, and rare genetic disorder management requires advanced quality monitoring systems. The worldwide market growth receives support from the need to maintain uniform product quality, together with enhanced regulatory oversight of mRNA production methods.
The market size for mRNA quality monitoring experiences major changes because of technological advancements in the industry. Scientists now perform more precise and efficient high-throughput mRNA quality analysis through the combined use of chromatography, mass spectrometry, next-generation sequencing (NGS), and capillary electrophoresis. The integration of automation systems with artificial intelligence technology in analytical operations leads to better accuracy results and reduced operational costs. The United States, along with Europe and Asia-Pacific countries, allocate funding through government programs to establish advanced analytical facilities. These support vaccine development, biomanufacturing quality standards, and biotechnology innovation. The regulatory bodies and biotech companies work together to establish mRNA standards and quality assurance systems, which support ongoing market development.
Product & Service Insights
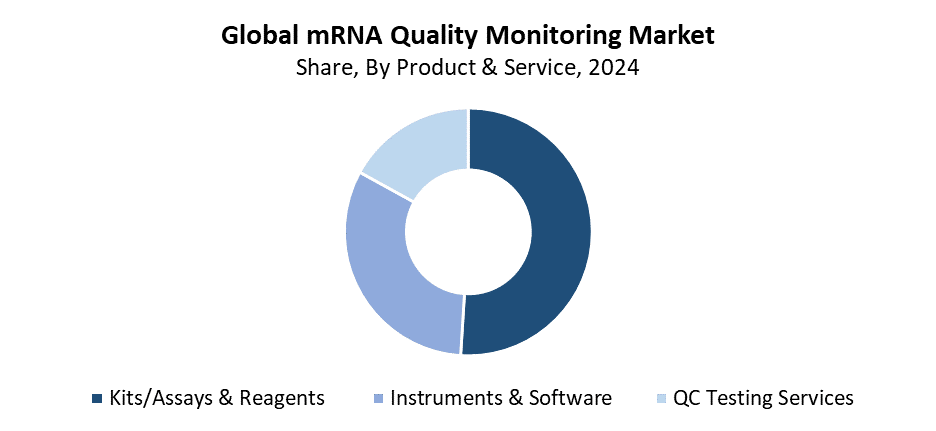
The kits/assays & reagents segment held the largest revenue share of 51.82% in 2024, and led the mRNA quality monitoring market. The main factor behind its dominance is the rising need for mRNA analysis solutions that are dependable, high-quality, and ready to use during the R&D and manufacturing stages. The evaluation of mRNA purity and integrity, concentration and stability requires these kits and reagents to maintain product consistency and meet regulatory standards. The development of mRNA-based vaccines and treatments has accelerated the usage of specialised analytical reagents in the field. The global market dominance of kits/assays & reagents continues to grow because of continuous product advancements and user-friendly solutions, and expanding specialised assay kit availability.
The QC testing services segment of the mRNA quality monitoring market is expected to grow at the fastest CAGR during the forecasted period. The fast market expansion results from biotech and pharmaceutical companies outsourcing quality control operations to specialised service providers who deliver both affordable and scalable high-precision mRNA testing solutions. QC testing services provide multiple analytical techniques, which include mass spectrometry, chromatography, sequencing, and stability testing. The methods serve as vital tools for protecting mRNA effectiveness and purity and for upholding regulatory standards. The worldwide market expansion continues because new biotech companies require mRNA vaccines and treatments and need to meet strict quality standards. They lack internal testing capabilities.
Technology Insights
The electrophoresis segment led the mRNA quality monitoring market in 2024 by holding the largest revenue share of 32.16%. Electrophoresis techniques have become the leading method because they allow researchers to assess mRNA integrity and size, and purity. These are vital for both scientific studies and industrial production. The high-resolution separation, dependable results, and low operating costs of electrophoresis make it the preferred quality testing method for laboratories and manufacturing facilities. The market maintains its leading position because mRNA vaccines and treatments continue to gain popularity, while quality monitoring devices maintain strong demand. The market position of mRNA quality monitoring has become more dominant because automated high-throughput electrophoresis devices now provide better accuracy and speed in their operations.
The LC-MS segment of the mRNA quality monitoring market is expected to grow at the fastest CAGR throughout the forecast period. The demand for precise and sensitive mRNA analysis, which includes sequence verification and purity assessment, and detection of impurities and alterations, drives this market expansion. The creation of vaccines and treatments under strict regulatory environments requires exact analysis of mRNA quality characteristics through LC-MS technology. The combination of high-resolution LC-MS instrument development with automated systems and bioinformatics tool integration has enhanced both throughput and accuracy. This explains why biotech and pharmaceutical companies worldwide adopt this technology at a fast pace. The market is growing at a fast pace because it serves as a tool for research operations and quality control procedures.
Regional Insights
North America led the mRNA quality monitoring market with the largest revenue share of 35.13% in 2024. The rapid acceptance of mRNA-based treatments and vaccines after the COVID-19 pandemic has become the leading cause for this market dominance because it drives increased funding toward mRNA research and production. The area benefits from strong R&D capabilities, a large presence of top biotechnology and pharmaceutical companies, and a sophisticated healthcare infrastructure. The market expansion receives additional support from government initiatives, which protect quality standards. Major financial backing for biopharmaceutical research and development also supports the market. The extensive availability of cutting-edge analytical tools, including LC-MS, electrophoresis, and high-throughput QC testing services, also helps explain why North America dominates the global market for mRNA quality monitoring.
Europe mRNA Quality Monitoring Market Trends
The market for mRNA quality monitoring market size in Europe experiences significant growth because mRNA-based vaccines and treatments have become more widely used throughout the continent. The market experiences growth because biotechnology research receives more funding and pharmaceutical manufacturing capabilities improve, while quality control standards for mRNA production become more stringent. The strong biotech and pharmaceutical sectors, together with established healthcare systems of Germany, France, and the United Kingdom, drive these countries to lead the market. The precision and effectiveness of mRNA quality monitoring are being improved by technological developments in analytical instruments, including electrophoresis, LC-MS, and automated QC testing platforms. The European mRNA quality monitoring market receives additional support from government initiatives and funding programs for vaccine research, strict safety and quality regulations.
Asia Pacific mRNA Quality Monitoring Market Trends
The Asia Pacific mRNA quality monitoring market size is expected to grow at the fastest CAGR during the forecast period because the pharmaceutical and biotechnology industries are rapidly growing in China, India, and Japan. The market growth occurs because of increasing financial support for mRNA research and expanding application of mRNA vaccines and treatments, and growing requirements for top-quality production standards. The area receives advantages from an expanding number of patients who require advanced medical treatments, improved healthcare facilities, and convenient access to sophisticated analytical equipment. The Asia Pacific region leads the global mRNA quality monitoring market in growth speed because of government initiatives that support biopharmaceutical development and vaccine funding.
Key mRNA Quality Monitoring Companies:
The following are the leading companies in the mRNA quality monitoring market. These companies collectively hold the largest market share and dictate industry trends.
- Revvity, Inc.
- Creative Biolabs
- Merck KGaA
- Bio-Rad Laboratories, Inc.
- GenScript
- Waters Corporation
- TriLink BioTechnologies (Maravai LifeSciences)
- Agilent Technologies, Inc.
- Creative Diagnostics
- Vazyme International LLC
- Others
Recent Developments
- In July 2025, Sensible Biotechnologies teamed up with Sartorius Stedim Biotech to expand the production of Sensible's proprietary cell-based mRNA design and manufacturing platform.
- In February 2025, to examine the effectiveness of mRNA capping, Aldevron and InDevR partnered to test and validate chip-based technologies. The goal of this partnership was to help Aldevron's efforts in mRNA quality control and production optimisation by utilising InDevR's unique analytical platform to expedite and improve the accuracy of capping measures.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Decision Advisors has segmented the mRNA quality monitoring market based on the below-mentioned segments:
Global mRNA Quality Monitoring Market, By Product & Service
- Kits/Assays & Reagents
- Instruments & Software
- QC Testing Services
Global mRNA Quality Monitoring Market, By Technology
- LC-MS
- Electrophoresis
- ELISA
- PCR Techniques
- Other Technologies
Global mRNA Quality Monitoring Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa





