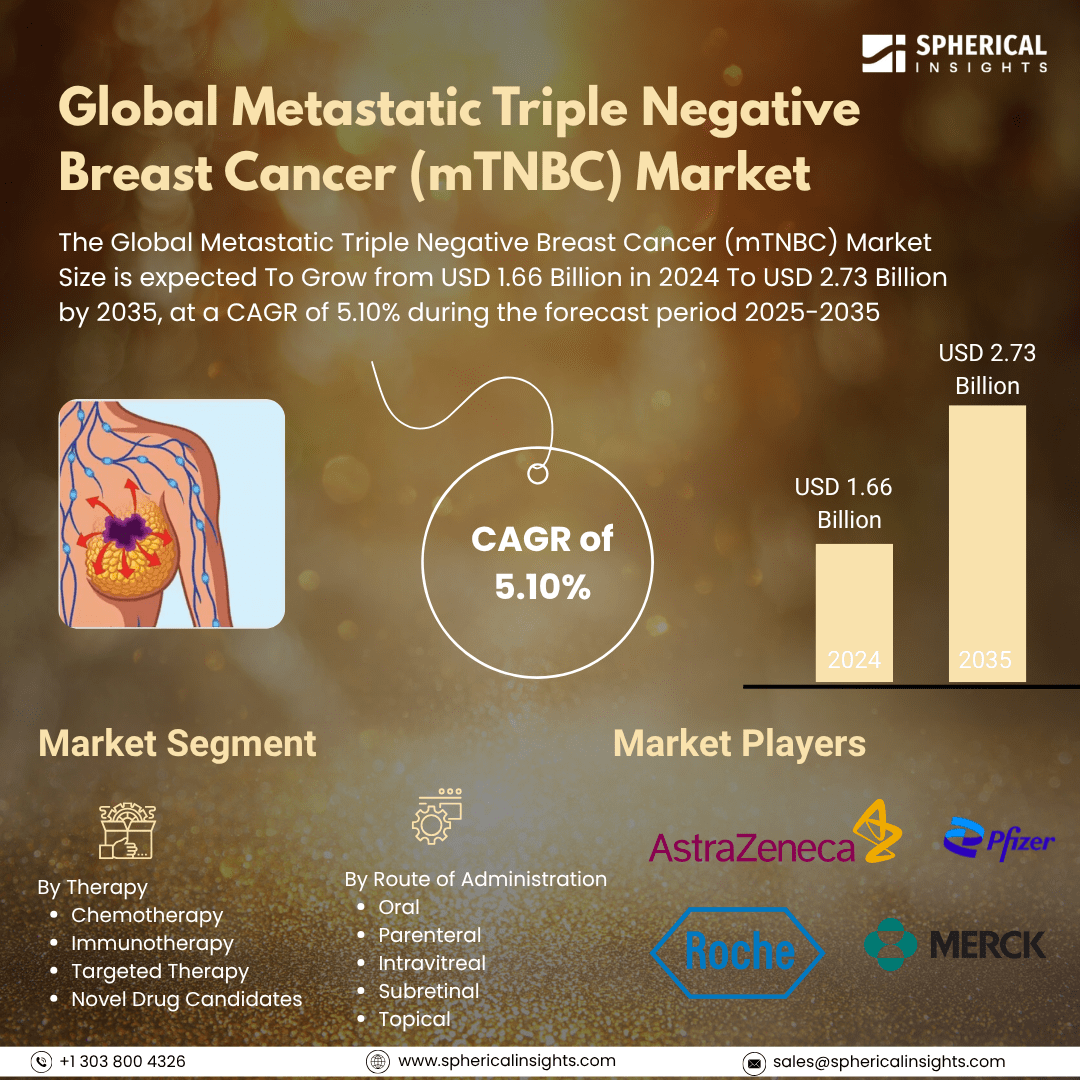
- As per Spherical Insights & Consulting, The Global Metastatic Triple Negative Breast Cancer (mTNBC) Market Size is expected To Grow from USD 1.66 Billion in 2025 to USD 2.73 Billion by 2035, at a CAGR of 5.10% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Metastatic Triple Negative Breast Cancer (mTNBC) Market Companies such as AstraZeneca, Pfizer, Roche, Merck and Co., Novartis, Bristol-Myers Squibb, Eli Lilly, Sanofi, GlaxoSmithKline, Johnson and Johnson, Amgen, Celgene, AbbVie, Gilead Sciences, Astellas Pharma, and Others
Metastatic Triple Negative Breast Cancer (mTNBC) Treatment Market: Understanding and Treatment Algorithm:
metastatic Triple Negative breast cancer (mTNBC) is an aggressive breast cancer subtype that lacks estrogen, progesterone, and HER2 receptors. It spreads beyond the breast to other body parts, making treatment challenging. Due to limited targeted therapies, mTNBC often requires chemotherapy and emerging immunotherapies for management.
Metastatic Triple Negative Breast Cancer (mTNBC) Diagnosis:
Diagnosis of metastatic Triple Negative breast cancer (mTNBC) involves imaging tests like CT, MRI, and PET scans to detect metastasis, along with biopsy and immunohistochemistry (IHC) to confirm the absence of estrogen, progesterone, and HER2 receptors. Accurate diagnosis guides appropriate treatment strategies for mTNBC patients.
Metastatic Triple Negative Breast Cancer (mTNBC) Treatment
Treatment of metastatic Triple Negative breast cancer (mTNBC) primarily includes chemotherapy to target rapidly dividing cancer cells. Recent advances involve immunotherapy and targeted therapies to improve outcomes. Due to its aggressive nature and lack of hormone receptors, treatment is often personalized and may include clinical trial options.
Metastatic Triple Negative Breast Cancer (mTNBC) Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Metastatic Triple Negative Breast Cancer (mTNBC), Gender-specific Diagnosed Incidence of Metastatic Triple Negative Breast Cancer (mTNBC), Type-specific Diagnosed Incidence of Metastatic Triple Negative Breast Cancer (mTNBC), Age-specific Diagnosed Incidence of Metastatic Triple Negative Breast Cancer (mTNBC), Diagnosed Incident Population based on Primary Site of Metastatic Triple Negative Breast Cancer (mTNBC), and Diagnosed Incident Population based on Histologic Classification of Metastatic Triple Negative Breast Cancer (mTNBC) Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of metastatic Triple Negative breast cancer (mTNBC) epidemiology in major markets worldwide.
Country Wise Metastatic Triple Negative Breast Cancer (mTNBC) Multiforme Epidemiology
- The epidemiology segment provides Metastatic Triple Negative Breast Cancer (mTNBC) prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Metastatic Triple Negative Breast Cancer (mTNBC) Recent Developments:
- In May 2025, Gilead Sciences announced that combining Trodelvy with Merck’s Keytruda reduced the risk of disease progression or death by 35% compared to Keytruda plus chemotherapy in first-line PD-L1+ metastatic Triple Negative breast cancer. The combination improved median progression-free survival to 11.2 months versus 7.8 months.
Metastatic Triple Negative Breast Cancer (mTNBC) Marketed Drugs:
- Trodelvy: Gilead Sciences
Trodelvy (sacituzumab govitecan-hziy) is an antibody-drug conjugate targeting Trop-2. It delivers a cytotoxic agent directly to cancer cells and is FDA-approved for adult patients with unresectable locally advanced or metastatic triple negative breast cancer who have received two or more prior systemic therapies.
Keytruda (pembrolizumab) is an anti-PD-1 monoclonal antibody that enhances immune response against tumor cells. It is FDA-approved in combination with chemotherapy for first-line treatment of PD-L1-positive metastatic triple negative breast cancer based on PD-L1 expression levels.
Tecentriq (atezolizumab) is an anti-PD-L1 checkpoint inhibitor approved for use in combination with nab-paclitaxel for PD-L1 positive mTNBC. It works by blocking the interaction between PD-L1 and its receptors, enhancing the immune system's ability to fight cancer.
Metastatic Triple Negative Breast Cancer (mTNBC): Emerging Therapies
- BNT327: It is a bispecific antibody in late-stage trials for mTNBC. It targets two immune checkpoints to enhance T-cell activation and tumor response, aiming to improve outcomes in patients resistant to standard PD-1/PD-L1 therapies.
- Datopotamab deruxtecan: It is an antibody-drug conjugate targeting TROP2, currently in advanced trials for mTNBC. It is designed to selectively deliver chemotherapy to tumor cells, potentially improving efficacy while minimizing systemic toxicity.
- IMMU-132 (Sacituzumab govitecan): Though now approved as Trodelvy, it remains under investigation for expanded indications and combinations in mTNBC. It delivers SN-38, a topoisomerase I inhibitor, directly to cancer cells expressing Trop-2.
- ALX148: It is a CD47-blocking fusion protein being evaluated in combination with chemotherapy and checkpoint inhibitors for mTNBC. It is designed to enhance anti-tumor immune response by enabling macrophage-mediated phagocytosis of cancer cells.
Metastatic Triple Negative Breast Cancer (mTNBC) Market Outlook
- The metastatic Triple Negative breast cancer (mTNBC) market comprises therapies and diagnostics developed specifically for treating advanced-stage breast cancers lacking estrogen,progesterone, and HER2 receptors. It focuses on innovative treatment approaches like chemotherapy, immunotherapy, and targeted agents to manage this aggressive and hard-to-treat cancer subtype.
- Key drivers of the metastatic Triple Negative breast cancer (mTNBC) market include rising incidence of aggressive breast cancers, increased awareness and early detection, advancements in immunotherapy and targeted treatments, growing investment in oncology research, and strong demand for effective therapies due to limited existing treatment options.
Metastatic Triple Negative Breast Cancer (mTNBC) Market Segmentation
By Therapy:
- Chemotherapy
- Immunotherapy
- Targeted Therapy
- Novel Drug Candidates
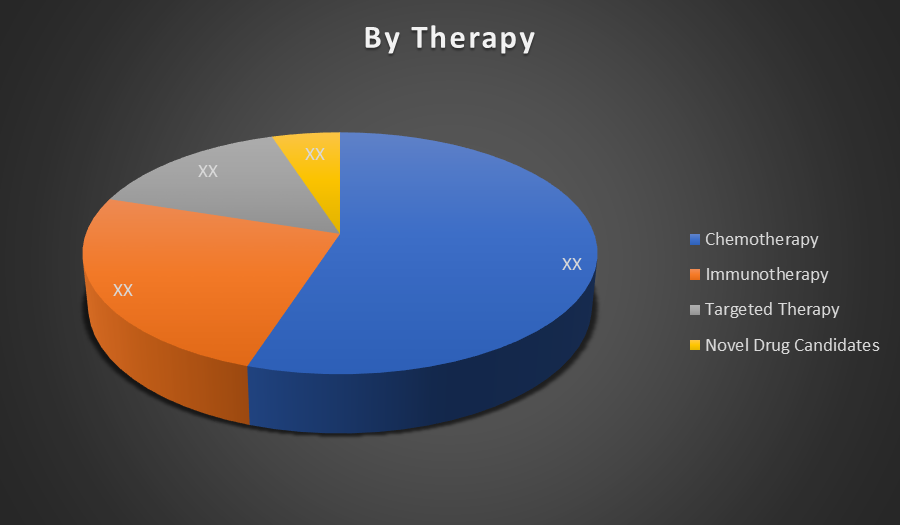
The Chemotherapy segment holds the largest share in the global metastatic Triple Negative breast cancer (mTNBC) market. This is because it remains the first line treatment for most patients due to the aggressive nature of mTNBC and the lack of hormone or HER2 targets for other therapies.
By Route of Administration:
- Oral
- Parenteral
- Intravitreal
- Subretinal
- Topical
The Parenteral route holds the largest share in the global metastatic Triple Negative breast cancer (mTNBC) market. Most approved therapies, including chemotherapy, immunotherapy, and antibody-drug conjugates, are administered intravenously due to the need for rapid, systemic drug delivery in treating this aggressive and fast-spreading cancer.
Regional Segment Analysis of the Metastatic Triple Negative Breast Cancer (mTNBC) Market
North America holds the largest share of the metastatic triple negative breast cancer (mTNBC) market. This is primarily due to the high incidence of breast cancer, widespread awareness, and access to advanced diagnostic and therapeutic options. The region benefits from strong healthcare infrastructure, significant funding for oncology research, and early regulatory approvals by the FDA for innovative therapies like immunotherapy and antibody-drug conjugates. Additionally, the presence of major pharmaceutical players and a high rate of clinical trial activity contribute to the region’s dominance.
The Asia-Pacific region is the fastest-growing market for mTNBC, driven by rising cancer prevalence, improving healthcare infrastructure, and expanding access to cancer treatments. Countries like China, India, and Japan are investing heavily in cancer screening programs, patient awareness, and precision medicine. Increasing collaborations between global pharma companies and regional healthcare providers, along with a large and aging population, are accelerating clinical adoption of advanced therapies. Furthermore, growing participation in global clinical trials is expanding the reach of novel treatments in the region.
Metastatic Triple Negative Breast Cancer (mTNBC) Market Key Companies
- AstraZeneca
- Pfizer
- Roche
- Merck and Co.
- Novartis
- Bristol-Myers Squibb
- Eli Lilly
- Sanofi
- GlaxoSmithKline
- Johnson and Johnson
- Amgen
- Celgene
- AbbVie
- Gilead Sciences
- Astellas Pharma
- Others
Metastatic Triple Negative Breast Cancer (mTNBC) Therapeutics Market Report Scope
- The Metastatic Triple Negative Breast Cancer (mTNBC) therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Metastatic Triple Negative Breast Cancer (mTNBC)’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Metastatic Triple Negative Breast Cancer (mTNBC) therapies is provided, including an evaluation of new treatments expected to influence the current Metastatic Triple Negative Breast Cancer (mTNBC) treatment market landscape.
- The report includes a detailed review of the Metastatic Triple Negative Breast Cancer (mTNBC) therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Metastatic Triple Negative Breast Cancer (mTNBC) Market Forecasting report offers valuable insights into trends shaping the global Metastatic Triple Negative Breast Cancer (mTNBC) market, helping to develop effective business strategies.
Metastatic Triple Negative Breast Cancer (mTNBC) Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Metastatic Triple Negative Breast Cancer (mTNBC) Therapeutic Approaches in Metastatic Triple Negative Breast Cancer (mTNBC)
- Review Of Drugs in Development for Metastatic Triple Negative Breast Cancer (mTNBC)
- Market, Growth, and Trends in Metastatic Triple Negative Breast Cancer (mTNBC)
- Market Opportunities in Metastatic Triple Negative Breast Cancer (mTNBC) Treatment
- Effects Of Future Therapies on Metastatic Triple Negative Breast Cancer (mTNBC) Treatment.
Metastatic Triple Negative Breast Cancer (mTNBC) Treatment Market Report Key Strengths
- 15 Years Metastatic Triple Negative Breast Cancer (mTNBC) Market Forecast
- Global Coverage
- Metastatic Triple Negative Breast Cancer (mTNBC) Epidemiology Segmentation
- Key Cross Competition
Metastatic Triple Negative Breast Cancer (mTNBC) Treatment Market Report Assessment
- Present Practices in the Metastatic Triple Negative Breast Cancer (mTNBC) Treatment Market
- Review of Investigational Metastatic Triple Negative Breast Cancer (mTNBC) Drugs
- Attractiveness of the Metastatic Triple Negative Breast Cancer (mTNBC) Drug Market
- Metastatic Triple Negative Breast Cancer (mTNBC) Market Drivers
- Metastatic Triple Negative Breast Cancer (mTNBC) Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the metastatic Triple Negative breast cancer (mTNBC) market based on the following segments:
Global Metastatic Triple Negative Breast Cancer (mTNBC) Market, By Therapy
- Chemotherapy
- Immunotherapy
- Targeted Therapy
- Novel Drug Candidates
Global Metastatic Triple Negative Breast Cancer (mTNBC) Market, By Route of Administration
- Oral
- Parenteral
- Intravitreal
- Subretinal
- Topical
Global Metastatic Triple Negative Breast Cancer (mTNBC) Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa