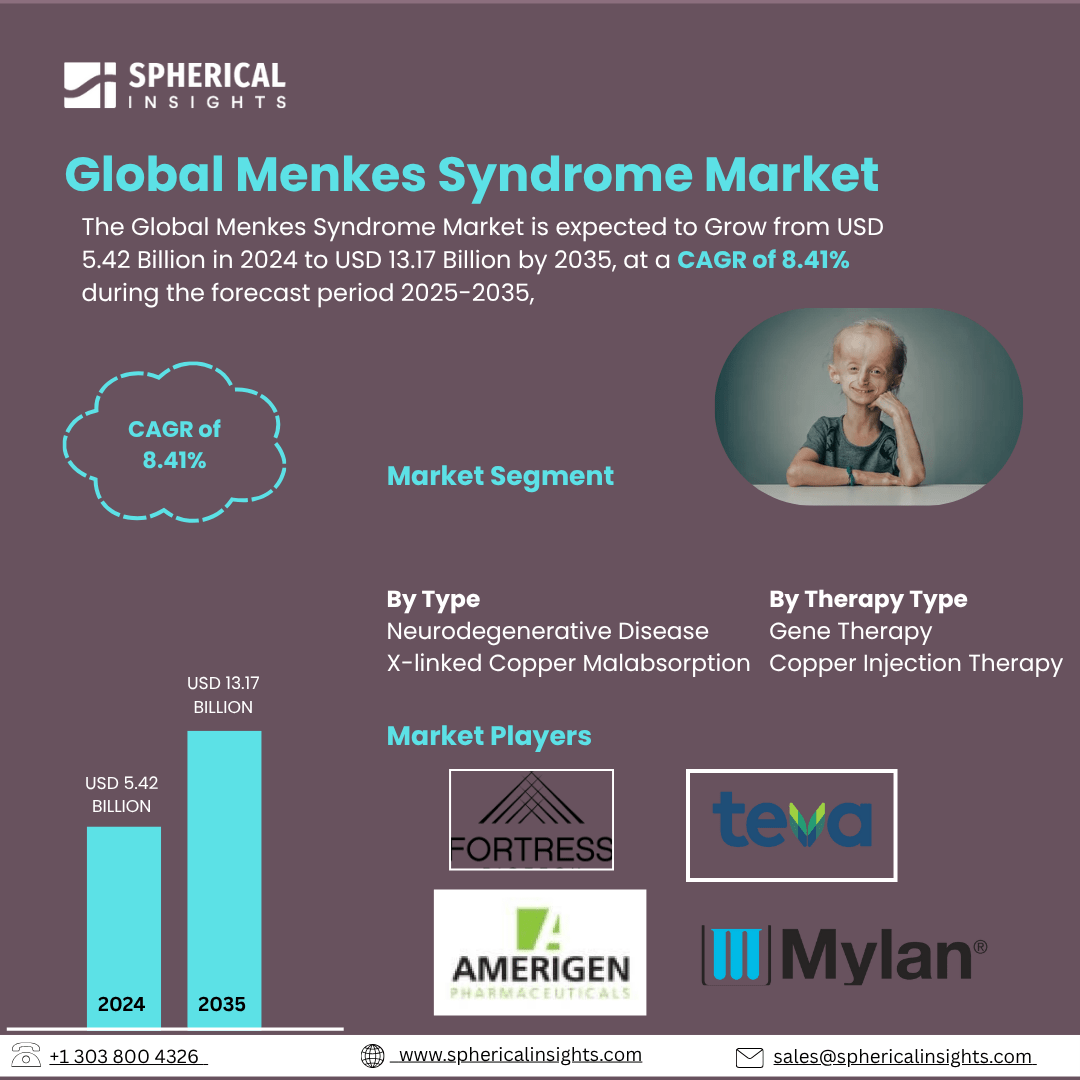
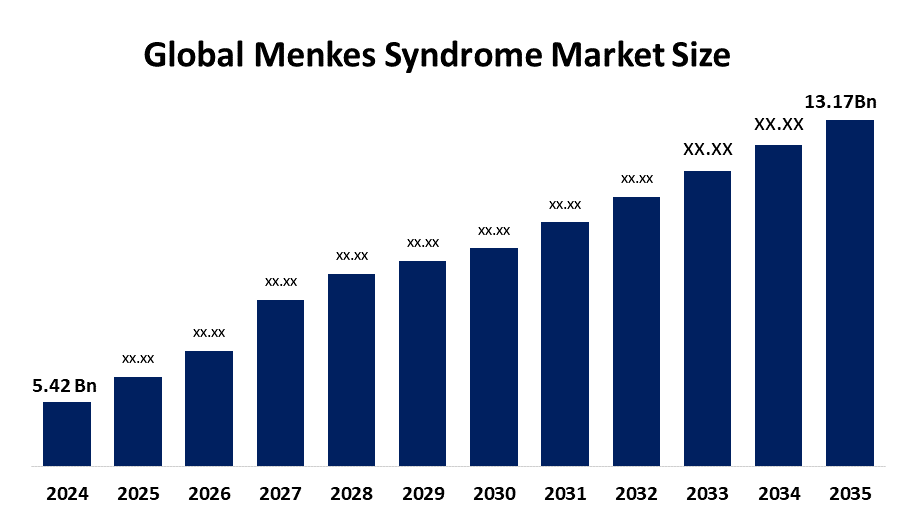
- As per Spherical Insights & Consulting, The Global Menkes Syndrome Market Size is expected to Grow from USD 5.42 Billion in 2024 to USD 13.17 Billion by 2035, at a CAGR of 8.41% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Menkes Syndrome Market Companies such as Fortress Biotech, Teva Pharmaceutical Industries Ltd, Amerigen Pharmaceuticals Limited, Mylan N.V., Bausch Health, H. Lundbeck A/S, Cyprium Therapeutics, Ultragenyx Pharmaceutical Inc., BioMarin Pharmaceutical, Alexion Pharmaceuticals, Neurocrine Biosciences, Retrophin, Inc., Neurotrope Bioscience, Inc., Stem Cells, Inc., Strongbridge Biopharma plc., and Others.
Menkes Syndrome Treatment Market: Understanding and Treatment Algorithm:
Menkes Syndrome is a rare, genetic disorder caused by mutations in the ATP7A gene, leading to impaired copper absorption and distribution in the body. This results in severe neurological degeneration, developmental delays, and connective tissue abnormalities. It primarily affects males and typically presents in infancy with progressive and often fatal symptoms.
Menkes Syndrome Diagnosis:
Diagnosis of Menkes Syndrome involves a combination of clinical evaluation, blood and hair analysis, and genetic testing. Low copper and ceruloplasmin levels in the blood, characteristic kinky hair, and ATP7A gene mutation confirmation help establish the diagnosis. Early detection is crucial for initiating timely copper-based treatments to improve outcomes.
Menkes Syndrome Treatment
Treatment for Menkes Syndrome focuses on early copper supplementation through injections to correct copper deficiency and slow disease progression. Supportive therapies include managing neurological symptoms and improving quality of life. Gene therapy and novel experimental treatments are being explored but are not yet widely available.
Menkes Syndrome Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Menkes Syndrome, Gender-specific Diagnosed Incidence of Menkes Syndrome, Type-specific Diagnosed Incidence of Menkes Syndrome, Age-specific Diagnosed Incidence of Menkes Syndrome, Diagnosed Incident Population based on Primary Site of Menkes Syndrome, and Diagnosed Incident Population based on Histologic Classification of Menkes Syndrome Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of Menkes Syndrome epidemiology in major markets worldwide.
Country Wise- Menkes Syndrome Multiforme Epidemiology
- The epidemiology segment provides Menkes Syndrome prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Menkes Syndrome Recent Developments:
- In January 2025, Fortress Biotech and Cyprium Therapeutics announced that the U.S. FDA accepted their New Drug Application (NDA) for CUTX-101 (Copper Histidinate) to treat Menkes disease. The NDA submission was supported by positive clinical efficacy results, demonstrating a nearly 80% reduction in the risk of death for patients receiving early treatment with CUTX-101 compared to an untreated historical control cohort.
Menkes Syndrome Marketed Drugs:
- CUTX-101: Cyprium Therapeutics
CUTX-101 (Copper Histidinate) is an FDA-designated orphan drug developed for treating Menkes Syndrome. It provides copper supplementation to address the genetic copper transport defect, aiming to improve survival and neurological outcomes in affected infants. It has received Breakthrough Therapy and Fast Track designations.
- Copper Histidinate Injection: Various Manufacturers
Copper Histidinate injection is used as a standard treatment to supply copper in Menkes patients, improving copper-dependent enzyme function and neurological development. It is administered intravenously shortly after diagnosis to mitigate disease progression.
- Copper Chloride: Various Manufacturers
Copper chloride supplements have been used off-label for Menkes Syndrome to increase copper levels in the body. Though less commonly used than copper histidinate, it aims to support essential enzymatic activities impaired in the disorder.
Menkes Syndrome: Emerging Therapies
- AT-001: It is an investigational gene therapy in early clinical trials aimed at correcting ATP7A gene mutations that cause Menkes Syndrome. It seeks to restore proper copper transport and reduce neurological damage, potentially offering a disease-modifying treatment.
- AAV-ATP7A: It is an adeno-associated virus-based gene therapy designed to deliver a functional copy of the ATP7A gene to patients with Menkes Syndrome. It aims to address the root cause by restoring copper metabolism and improving survival rates.
- CUTX-101 Combination Therapy: Emerging combination therapies involving CUTX-101 and novel gene therapies are being explored to enhance treatment efficacy by simultaneously supplying copper and correcting genetic defects in Menkes patients.
- BMX-101: It is an experimental antioxidant therapy in preclinical stages aimed at reducing oxidative stress and neurological damage in Menkes Syndrome patients, potentially complementing existing copper supplementation treatments.
Menkes Syndrome Market Outlook
- The Menkes Syndrome market refers to the global industry focused on developing, manufacturing, and distributing treatments and therapies for Menkes disease, a rare genetic disorder causing copper deficiency. The market includes pharmaceuticals, gene therapies, and diagnostic tools aimed at improving patient outcomes and managing symptoms.
- Key drivers of the market include increasing awareness and early diagnosis, advancements in gene therapy and copper supplementation treatments, growing investment in rare disease research, and supportive regulatory frameworks encouraging orphan drug development to address the unmet medical needs of affected patients.
- Opportunities in the market arise from ongoing research in gene editing and novel therapies, expanding healthcare infrastructure in emerging regions, potential for personalized medicine, and increasing collaboration between biotech firms and research institutions to develop effective, targeted treatments for this rare disorder.
- Governments worldwide have launched initiatives supporting rare disease research, providing funding for Menkes Syndrome studies, promoting early diagnosis programs, and implementing orphan drug policies to encourage the development and accessibility of specialized treatments for patients with this rare genetic disorder.
- Limited awareness and diagnosis delays hinder effective treatment and market growth for Menkes Syndrome therapies.
- Rising investments in gene therapy and growing adoption of early diagnostic techniques drive the Menkes Syndrome market growth.
Menkes Syndrome Market Segmentation
By Type:
- Neurodegenerative Disease
- X-linked Copper Malabsorption
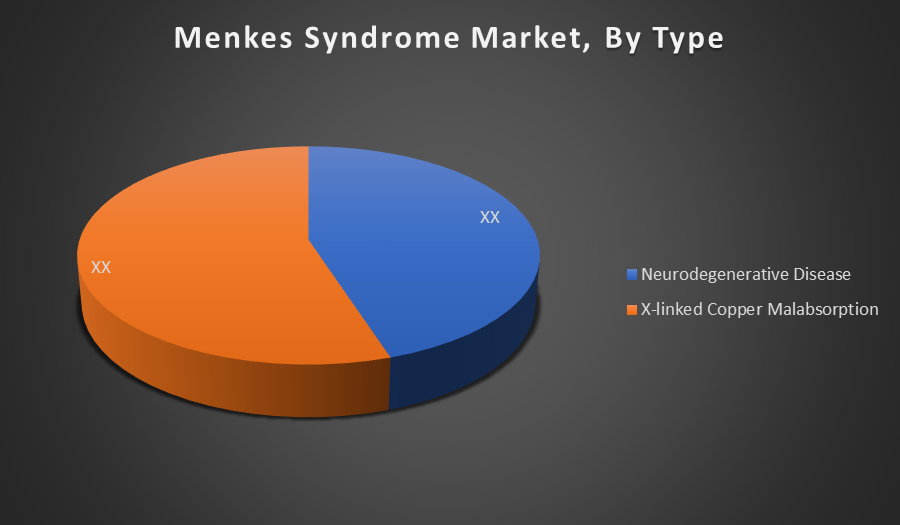
The X-linked Copper Malabsorption segment holds the largest share in the Menkes Syndrome market due to its direct association with the genetic cause of the disease. It includes the majority of diagnosed cases, leading to higher demand for targeted therapies like copper supplementation and gene-based treatments.
By Therapy Type:
- Gene Therapy
- Copper Injection Therapy
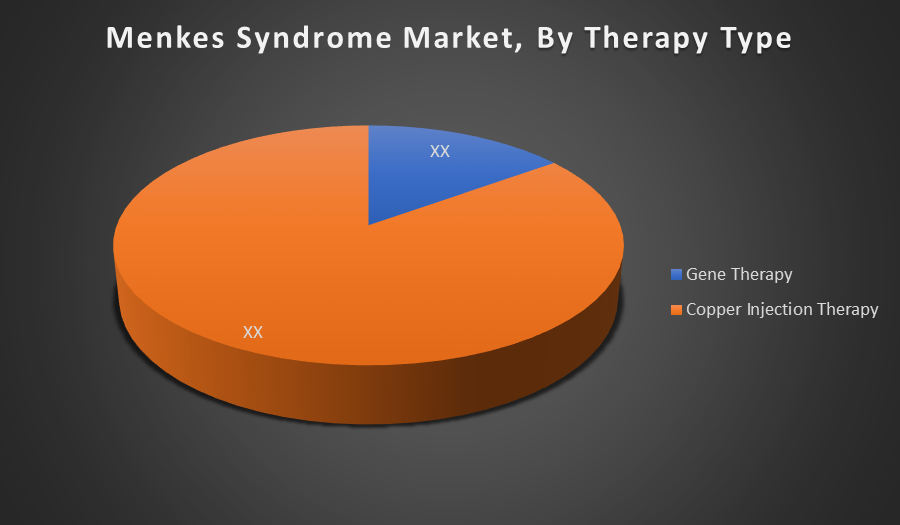
Copper Injection Therapy holds the largest share in the Menkes Syndrome market, primarily because it is the current standard of care. It is widely used for early treatment to manage copper deficiency, while gene therapy remains in clinical stages with limited accessibility and regulatory approvals.
Regional Segment Analysis of the Menkes Syndrome Market
North America leads the Menkes Syndrome market due to its strong healthcare infrastructure, high public and private investment in rare disease research, and faster regulatory pathways like the FDA’s Orphan Drug and Breakthrough Therapy designations. The U.S. is home to key players such as Fortress Biotech and Cyprium Therapeutics, which are spearheading clinical trials and therapy development, including CUTX-101 and gene therapies. Widespread access to newborn screening, early copper injection interventions, and awareness campaigns also contribute to better diagnosis and management rates in this region, reinforcing its dominant market position.
Asia-Pacific is witnessing the fastest growth in the Menkes Syndrome market, fueled by rising healthcare expenditures, improving access to advanced diagnostics, and a growing focus on rare genetic disorders. Countries like China, Japan, and India are increasingly investing in research collaborations and healthcare infrastructure. Expanding patient registries, government-backed rare disease programs, and increased awareness among clinicians are improving early diagnosis and treatment access.
Menkes Syndrome Market Key Companies
- Fortress Biotech
- Teva Pharmaceutical Industries Ltd
- Amerigen Pharmaceuticals Limited
- Mylan N.V.
- Bausch Health
- H. Lundbeck A/S
- Cyprium Therapeutics
- Ultragenyx Pharmaceutical Inc.
- BioMarin Pharmaceutical
- Alexion Pharmaceuticals
- Neurocrine Biosciences
- Retrophin, Inc.
- Neurotrope Bioscience, Inc.
- Stem Cells, Inc.
- Strongbridge Biopharma plc.
- Others
Menkes Syndrome Therapeutics Market Report Scope
- The Menkes Syndrome therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Menkes Syndrome’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Menkes Syndrome therapies is provided, including an evaluation of new treatments expected to influence the current Menkes Syndrome treatment market landscape.
- The report includes a detailed review of the Menkes Syndrome therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Menkes Syndrome Market Forecasting report offers valuable insights into trends shaping the global Menkes Syndrome market, helping to develop effective business strategies.
Menkes Syndrome Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Menkes Syndrome Therapeutic Approaches in Menkes Syndrome
- Review Of Drugs in Development for Menkes Syndrome
- Market, Growth, and Trends in Menkes Syndrome
- Market Opportunities in Menkes Syndrome Treatment
- Effects Of Future Therapies on Menkes Syndrome Treatment.
Menkes Syndrome Treatment Market Report Key Strengths
- 15 Years Menkes Syndrome Market Forecast
- Global Coverage
- Menkes Syndrome Epidemiology Segmentation
- Key Cross Competition
Menkes Syndrome Treatment Market Report Assessment
- Present Practices in the Menkes Syndrome Treatment Market
- Review of Investigational Menkes Syndrome Drugs
- Attractiveness of the Menkes Syndrome Drug Market
- Menkes Syndrome Market Drivers
- Menkes Syndrome Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Menkes Syndrome market based on the below-mentioned segments:
Global Menkes Syndrome Market, By Type
- Neurodegenerative Disease
- X-linked Copper Malabsorption
Global Menkes Syndrome Market, By Therapy Type
- Gene Therapy
- Copper Injection Therapy
Global Menkes Syndrome Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa