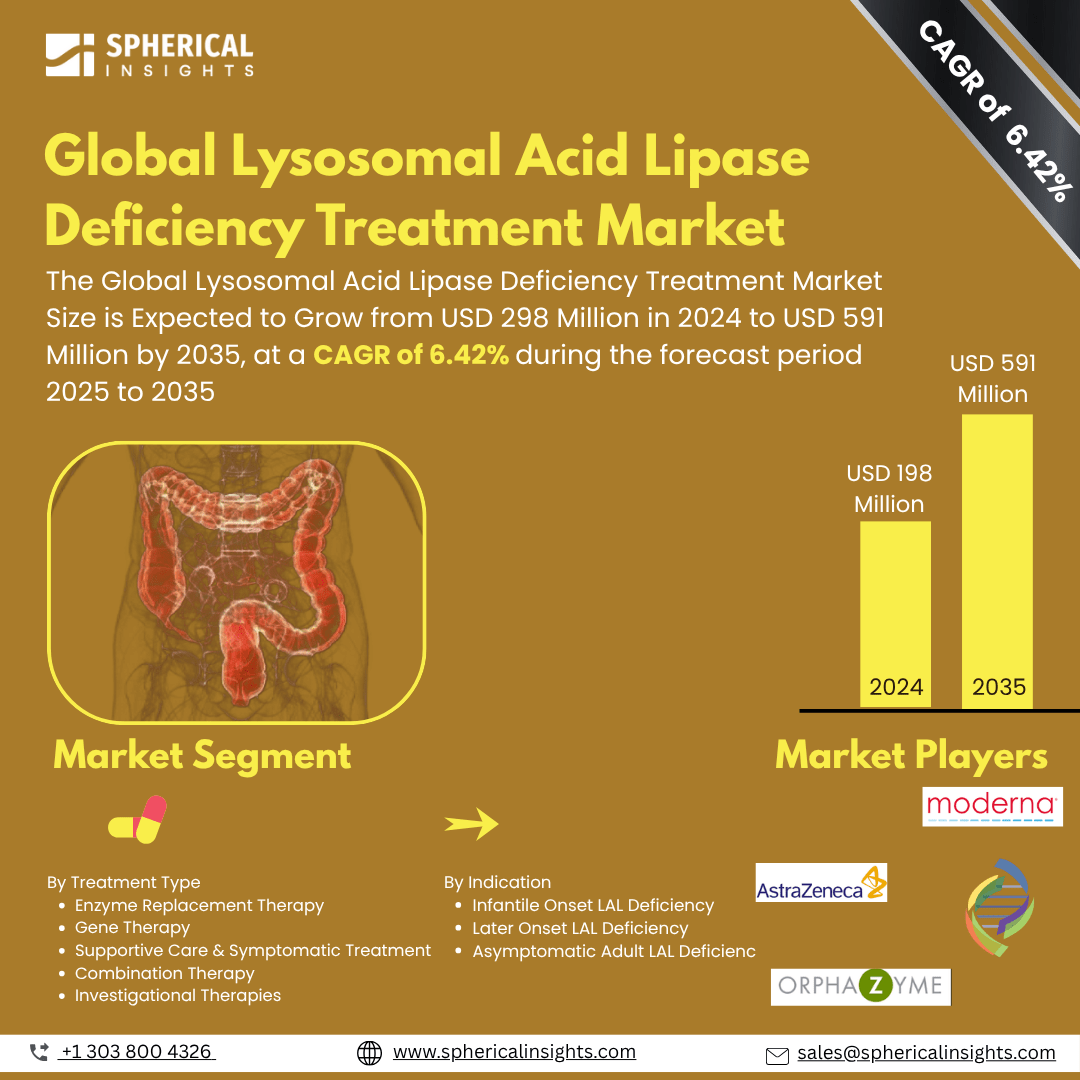
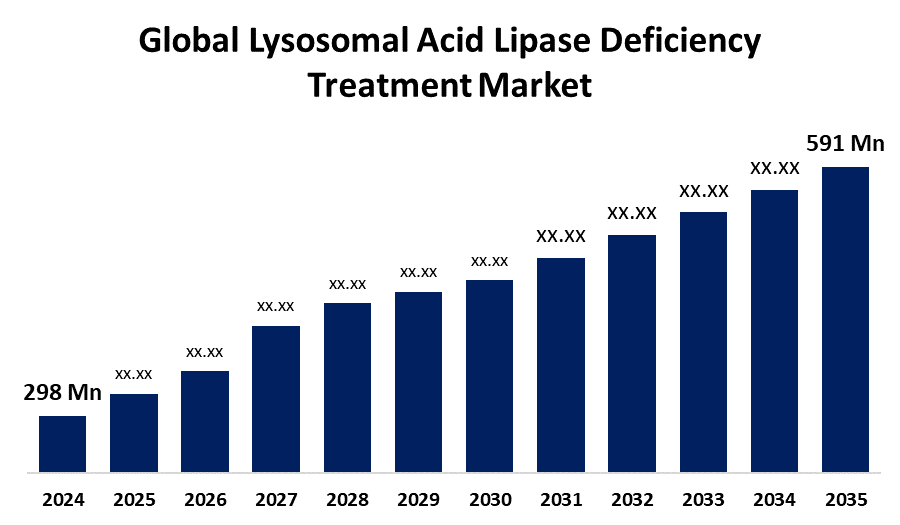
- As per Spherical Insights & Consulting, The Global Lysosomal Acid Lipase Deficiency Treatment Market Size is Expected to Grow from USD 298 Million in 2024 to USD 591 Million by 2035, at a CAGR of 6.42% during the forecast period 2025 to 2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Lysosomal Acid Lipase Deficiency Treatment Market Companies such as Vertex Pharmaceuticals, Alexion Pharmaceuticals, Orphazyme, Moderna, Chiesi Farmaceutici, AstraZeneca, Takeda Pharmaceutical, Enzo Biochem, BioMarin Pharmaceutical, Amryt Pharma, Regeneron Pharmaceuticals, Genzyme, LogicBio Therapeutics, Pfizer, Ultragenyx Pharmaceutical, and Others.
Lysosomal Acid Lipase Deficiency Treatment Market: Understanding and Treatment Algorithm:
Lysosomal Acid Lipase Deficiency (LALD) is a rare genetic disorder caused by mutations in the LIPA gene, leading to deficient activity of the lysosomal acid lipase enzyme. This results in harmful buildup of fats in organs like the liver, spleen, and blood vessels, causing progressive damage and multi organ complications.
Lysosomal Acid Lipase Deficiency Diagnosis:
Diagnosis of LALD involves blood tests to assess lipid levels, liver function tests, and enzyme activity assays. Genetic testing confirms mutations in the LIPA gene. Imaging studies and liver biopsies may also be used to evaluate organ damage. Early diagnosis is critical to prevent irreversible complications and initiate timely treatment.
Lysosomal Acid Lipase Deficiency Treatment:
Treatment for LALD primarily includes enzyme replacement therapy with sebelipase alfa, which restores deficient enzyme activity and reduces lipid buildup. Supportive care may include statins, dietary management, and monitoring of liver function. Emerging therapies such as gene therapy are being explored to provide long term or potentially curative solutions.
Lysosomal Acid Lipase Deficiency Treatment Epidemiology:
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Lysosomal Acid Lipase Deficiency Treatment, Gender specific Diagnosed Incidence of Lysosomal Acid Lipase Deficiency Treatment, Type specific Diagnosed Incidence of Lysosomal Acid Lipase Deficiency Treatment, Age specific Diagnosed Incidence of Lysosomal Acid Lipase Deficiency Treatment, Diagnosed Incident Population based on Primary Site of Lysosomal Acid Lipase Deficiency Treatment, and Diagnosed Incident Population based on Histologic Classification of Lysosomal Acid Lipase Deficiency Treatment Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights:
This section offers a global overview of Lysosomal Acid Lipase Deficiency Treatment epidemiology in major markets worldwide.
Country Wise Lysosomal Acid Lipase Deficiency Treatment Multiforme Epidemiology:
- The epidemiology segment provides Lysosomal Acid Lipase Deficiency Treatment prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Lysosomal Acid Lipase Deficiency Treatment Marketed Drugs:
• Kanuma: Alexion Pharmaceuticals
Kanuma is a recombinant human lysosomal acid lipase enzyme used as enzyme replacement therapy for LAL deficiency. It is FDA approved for both infantile- and later-onset forms of the disease. Kanuma helps reduce lipid accumulation in organs, improving liver function and survival rates, especially in pediatric patients.
• Sebelipase Alfa (Recombinant): AstraZeneca
Sebelipase Alfa is a biologic enzyme therapy designed to replace deficient LAL activity in patients with LAL deficiency. It breaks down cholesteryl esters and triglycerides inside cells, preventing organ damage. Administered intravenously, it is the first and only approved treatment specifically for this ultra-rare lysosomal storage disorder.
Lysosomal Acid Lipase Deficiency Treatment: Emerging Therapies:
- LAL GT 101: It is an investigational gene therapy in preclinical development for Lysosomal Acid Lipase Deficiency. It uses a recombinant AAV vector to deliver a functional LIPA gene, aiming to restore long-term enzyme activity. The therapy is designed to provide a one-time treatment alternative to lifelong enzyme replacement therapy.
- VX 264: It is a novel pipeline candidate exploring mRNA technology to address LAL deficiency at the genetic level. The therapy aims to deliver functional instructions for LAL enzyme production, enabling patients’ own cells to synthesize the enzyme. It is currently in early stage research as part of Vertex’s rare disease portfolio.
Lysosomal Acid Lipase Deficiency Treatment Market Outlook:
- The Lysosomal Acid Lipase Deficiency Treatment Market focuses on therapies aimed at managing a rare genetic disorder caused by the deficiency of the lysosomal acid lipase enzyme. Treatments include enzyme replacement therapy, gene therapy, and supportive care that help control lipid accumulation and prevent multi-organ complications.
- Key drivers include increasing diagnosis rates of rare metabolic disorders, rising awareness among healthcare professionals, and the availability of disease specific therapies like enzyme replacement. Regulatory support, orphan drug designations, and growing investments by biotech firms further contribute to market expansion across developed and emerging regions.
- Opportunities lie in the advancement of gene therapy, expansion into emerging markets, and development of oral or less invasive treatment options. Increasing clinical research and patient registry programs are helping identify more cases, enabling earlier treatment, and creating demand for innovative and accessible therapeutic approaches.
- Governments are supporting the market through rare disease funding, newborn screening programs, fast track approvals, and public-private research collaborations. These initiatives aim to improve early diagnosis, reduce disease burden, and accelerate the development of effective therapies for lysosomal storage disorders, including LAL deficiency.
- Limited awareness and delayed diagnosis hinder early treatment and reduce therapeutic impact.
- The market is projected to grow steadily due to the rising adoption of enzyme replacement and gene therapies, driven by improved access and early intervention strategies.
Lysosomal Acid Lipase Deficiency Treatment Market Segmentation:
By Treatment Type:
- Enzyme Replacement Therapy
- Gene Therapy
- Supportive Care & Symptomatic Treatment
- Combination Therapy
- Investigational Therapies
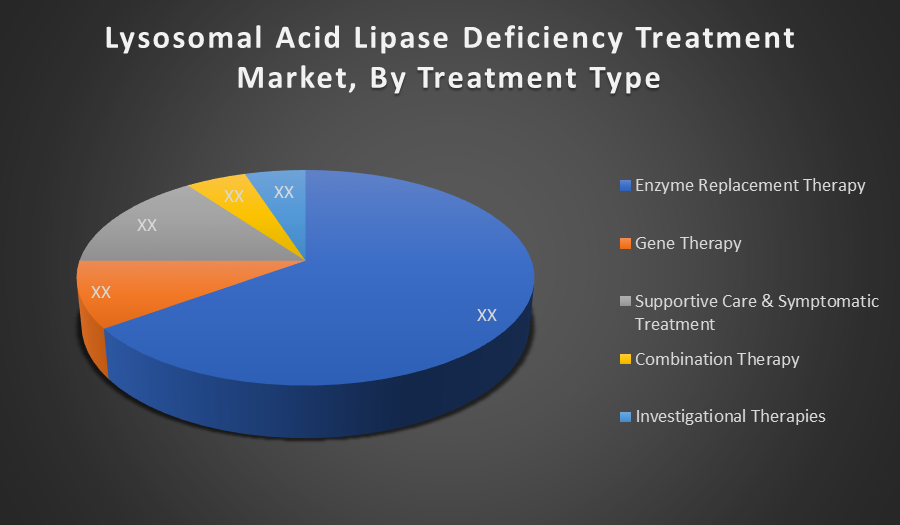
Enzyme Replacement Therapy (ERT) holds the largest market share due to its status as the only FDA approved, disease-specific treatment for LAL deficiency, Sebelipase alfa. It effectively replaces deficient lysosomal acid lipase, reducing disease progression. Its proven clinical efficacy and regulatory backing make it the cornerstone of LALD management.
By Indication:
- Infantile Onset LAL Deficiency
- Later Onset LAL Deficiency
- Asymptomatic Adult LAL Deficiency
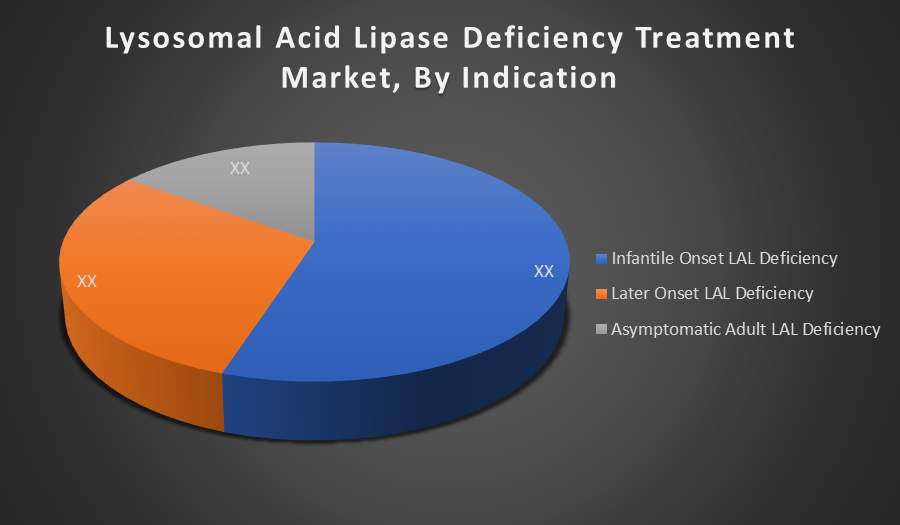
Infantile Onset LAL Deficiency leads the market share because it represents the most severe and life-threatening form of the disease, requiring immediate and continuous treatment. Infants often receive early diagnosis and intervention with ERT, driving higher demand in this segment compared to milder or asymptomatic adult forms of the disorder.
Regional Segment Analysis of the Lysosomal Acid Lipase Deficiency Treatment Market:
North America holds the largest share of the LAL Deficiency Treatment Market due to strong healthcare infrastructure, high diagnostic rates, and early adoption of innovative therapies like enzyme replacement. Regulatory support, presence of key pharmaceutical players, and access to rare disease funding contribute significantly. Additionally, increased awareness and newborn screening programs help drive consistent treatment demand across both infantile and later-onset patient groups.
Asia Pacific is the fastest growing region in the LAL Deficiency Treatment Market, driven by improving healthcare systems, rising awareness of rare diseases, and growing access to specialized treatments. Expansion of genetic testing, increasing government initiatives, and a large undiagnosed patient population are boosting market interest. Pharmaceutical companies are also targeting this region for clinical trials and strategic collaborations, accelerating market growth at a notable pace.
Lysosomal Acid Lipase Deficiency Treatment Market Key Companies:
- Vertex Pharmaceuticals
- Alexion Pharmaceuticals
- Orphazyme
- Moderna
- Chiesi Farmaceutici
- AstraZeneca
- Takeda Pharmaceutical
- Enzo Biochem
- BioMarin Pharmaceutical
- Amryt Pharma
- Regeneron Pharmaceuticals
- Genzyme
- LogicBio Therapeutics
- Pfizer
- Ultragenyx Pharmaceutical
- Others
Market Segment:
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Lysosomal Acid Lipase Deficiency Treatment market based on the following segments:
Global Lysosomal Acid Lipase Deficiency Treatment Market, By Treatment Type
- Enzyme Replacement Therapy
- Gene Therapy
- Supportive Care & Symptomatic Treatment
- Combination Therapy
- Investigational Therapies
Global Lysosomal Acid Lipase Deficiency Treatment Market, By Indication
- Infantile Onset LAL Deficiency
- Later Onset LAL Deficiency
- Asymptomatic Adult LAL Deficiency
Global Lysosomal Acid Lipase Deficiency Treatment Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa