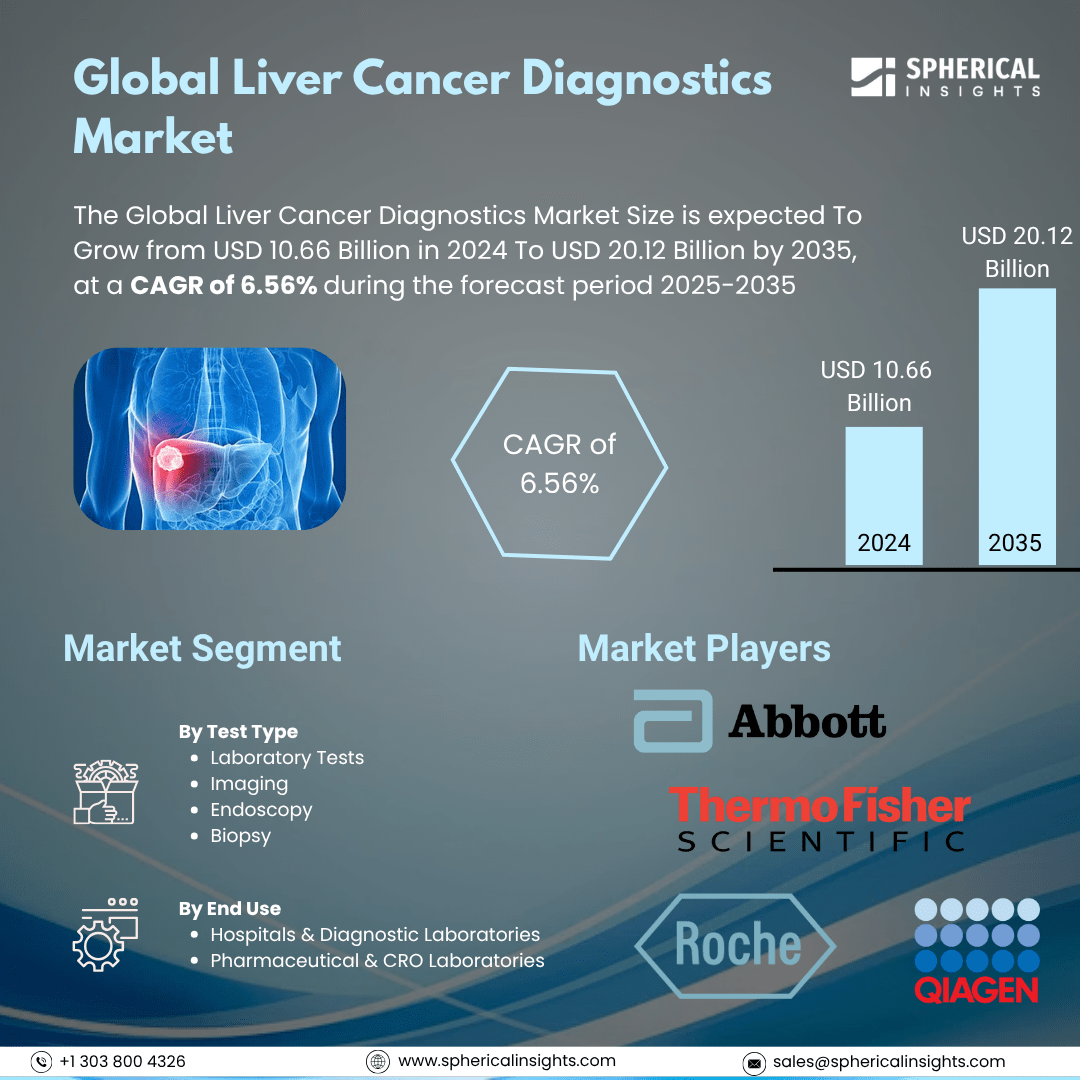
- As per Spherical Insights & Consulting, The Global Liver Cancer Diagnostics Market Size is expected To Grow from USD 10.66 Billion in 2024 to USD 20.12 Billion by 2035, at a CAGR of 6.56% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Liver Cancer Diagnostics Market Companies such as Abbott Laboratories, Thermo Fisher Scientific, F. Hoffmann La Roche, Qiagen N.V., Siemens Healthineers, Becton Dickinson & Company, Illumina, Epigenomics AG, Koninklijke Philips N.V., Fujifilm Medical Systems, Agilent Technologies, Danaher Corporation, Sysmex Corporation, Bio Rad Laboratories, Merck KGaA, and Others.
Liver Cancer Diagnostics Treatment Market: Understanding and Treatment Algorithm:
Liver cancer is a disease in which abnormal cells grow uncontrollably in the liver, often linked to chronic hepatitis B or C infections, alcohol abuse, or fatty liver disease. It can be aggressive and difficult to detect early, making timely diagnosis and treatment critical.
Liver Cancer Diagnosis:
Liver cancer diagnosis involves a combination of imaging tests, such as ultrasound, CT scans, and MRI, along with blood tests like alpha fetoprotein (AFP) to detect tumor markers. In some cases, a biopsy is performed to confirm malignancy and determine the cancer’s type and stage.
Liver Cancer Treatment
Liver cancer treatment depends on the stage and overall liver function. Common options include surgery, liver transplantation, and local ablation therapies. Advanced cases may require targeted therapies, immunotherapy, or chemotherapy. A multidisciplinary approach, involving oncologists, surgeons, and radiologists, is essential to personalize treatment and improve survival and quality of life for patients.
Liver Cancer Diagnostics Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Liver Cancer Diagnostics, Gender specific Diagnosed Incidence of Liver Cancer Diagnostics, Type specific Diagnosed Incidence of Liver Cancer Diagnostics, Age specific Diagnosed Incidence of Liver Cancer Diagnostics, Diagnosed Incident Population based on Primary Site of Liver Cancer Diagnostics, and Diagnosed Incident Population based on Histologic Classification of Liver Cancer Diagnostics Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of liver cancer diagnostics epidemiology in major markets worldwide.
Country Wise- Liver Cancer Diagnostics Multiforme Epidemiology
- The epidemiology segment provides Liver Cancer Diagnostics prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Liver Cancer Diagnostics: Recent Developments:
- In July 2023, Siemens Healthineers received FDA Breakthrough Device Designation for its Enhanced Liver Fibrosis (ELF) Test. This recognition highlights the test's potential to address unmet medical needs in diagnosing advanced fibrosis in patients with non-alcoholic fatty liver disease (NAFLD). The ELF Test offers a non-invasive alternative to biopsies, aiming to facilitate earlier intervention and improve patient outcomes.
Liver Cancer Diagnostics Marketed Drugs:
Keytruda is an anti PD 1 monoclonal antibody that enhances the immune system’s ability to fight cancer. It is FDA approved for unresectable or metastatic hepatocellular carcinoma in patients previously treated with sorafenib.
- Lenvima (lenvatinib): Eisai Co., Ltd.
Lenvima is a multikinase inhibitor approved for first line treatment of unresectable HCC. It targets multiple receptors involved in tumor angiogenesis and progression.
- Nexavar (sorafenib): Bayer AG
Nexavar was one of the first approved targeted therapies for advanced HCC. It works by inhibiting tumor cell proliferation and angiogenesis through multiple kinase pathways.
Liver Cancer Diagnostics: Emerging Therapies
- BNT327: It is a bispecific antibody in late-stage trials for liver cancer. It targets two immune checkpoints to boost T cell activity and improve tumor response, particularly in patients resistant to PD 1 or PD L1 therapies.
- TIL Therapy (Tumor Infiltrating Lymphocytes): This experimental immunotherapy involves harvesting and expanding a patient’s tumor-fighting immune cells. Currently under investigation for advanced liver cancers, it shows promise in improving immune recognition of tumors.
- CAR T Therapy (e.g., GPC3-CAR T cells): Targeting Glypican 3, a liver cancer-specific antigen, CAR T cell therapy is being studied to directly attack HCC cells. Early trials show potential for durable responses in advanced cases.
- HEPZATO (Delcath Systems): An investigational liver directed therapy that delivers high-dose chemotherapy directly to the liver while filtering blood to reduce systemic exposure. It is in late stage trials for liver dominant cancers, including HCC and metastases.
Liver Cancer Diagnostics Market Outlook
- The liver cancer diagnostics market refers to the segment of the healthcare industry focused on tools and technologies used to detect and diagnose liver cancer. This includes imaging modalities, laboratory tests, biopsy procedures, and emerging diagnostic innovations aimed at early detection, accurate staging, and monitoring of liver cancer progression or treatment response.
- Key drivers of the liver cancer diagnostics market include the rising global incidence of liver cancer, increasing awareness of early detection, and advancements in diagnostic technologies such as imaging, biomarkers, and liquid biopsy. Additionally, growing investments in oncology research and supportive healthcare policies further propel market growth.
- The liver cancer diagnostics market presents strong opportunities through the adoption of non invasive diagnostic tools like liquid biopsy and AI driven imaging. Expanding healthcare infrastructure in emerging economies, growing demand for early detection, and ongoing clinical research into novel biomarkers offer significant potential for innovation and market expansion.
- Government initiatives have prioritized early liver cancer detection through funding, screening, and improved diagnostic infrastructure. In the UK, NHS invited at risk individuals for mobile fibroscans and piloted community liver checks to identify advanced fibrosis. India’s NPCDCS integrates liver disease screening into primary healthcare facilities.
- A major challenge in liver cancer diagnostics is the late detection due to asymptomatic early stages and limited access to advanced diagnostic technologies in low resource settings.
- The liver cancer diagnostics market is projected to grow steadily due to increasing disease prevalence, technological advancements, and rising emphasis on early and accurate cancer detection.
Liver Cancer Diagnostics Market Segmentation
By Test Type:
- Laboratory Tests
- Imaging
- Endoscopy
- Biopsy
The Laboratory Tests segment holds the largest share in the global liver cancer diagnostics market. This dominance is attributed to their non invasive nature, cost-effectiveness, and ability to detect tumor markers such as alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP). Laboratory tests are essential for early detection, screening high-risk individuals, and monitoring disease progression. They are widely used in both clinical and research settings, contributing significantly to their market leadership.
By End Use:
- Hospitals & Diagnostic Laboratories
- Pharmaceutical & CRO Laboratories
The Hospitals & Diagnostic Laboratories segment holds the largest share in the global liver cancer diagnostics market. This is due to their extensive patient access, comprehensive diagnostic services, and ability to provide timely and accurate testing. Hospitals also serve as primary care centers, driving high demand for liver cancer diagnostic tests.
Regional Segment Analysis of the Liver Cancer Diagnostics Market
North America holds the largest share in the liver cancer diagnostics market, driven by well-established healthcare infrastructure, high awareness levels, widespread access to advanced diagnostic tools, and significant investment in research and development. The strong presence of leading diagnostic companies, robust reimbursement systems, and ongoing innovation in imaging and molecular testing further strengthens its dominance.
Asia-Pacific is experiencing the fastest growth in the liver cancer diagnostics market. This is due to the rising incidence of liver cancer, particularly in countries like China and India, coupled with increasing healthcare spending, government screening programs, and growing adoption of advanced diagnostic technologies across urban and rural settings. Expanding healthcare access, rising awareness, and local manufacturing support also contribute to accelerated market expansion.
Liver Cancer Diagnostics Market Key Companies
- Abbott Laboratories
- Thermo Fisher Scientific
- F. Hoffmann-La Roche
- Qiagen N.V.
- Siemens Healthineers
- Becton Dickinson & Company
- Illumina
- Epigenomics AG
- Koninklijke Philips N.V.
- Fujifilm Medical Systems
- Agilent Technologies
- Danaher Corporation
- Sysmex Corporation
- Bio-Rad Laboratories
- Merck KGaA
- Others
Liver Cancer Diagnostics Therapeutics Market Report Scope
- The Liver Cancer Diagnostics therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Liver Cancer Diagnostics’ epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Liver Cancer Diagnostics therapies is provided, including an evaluation of new treatments expected to influence the current Liver Cancer Diagnostics treatment market landscape.
- The report includes a detailed review of the Liver Cancer Diagnostics therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient Based Liver Cancer Diagnostics Market Forecasting report offers valuable insights into trends shaping the global Liver Cancer Diagnostics market, helping to develop effective business strategies.
Liver Cancer Diagnostics Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Liver Cancer Diagnostics Therapeutic Approaches in Liver Cancer Diagnostics
- Review Of Drugs in Development for Liver Cancer Diagnostics
- Market, Growth, and Trends in Liver Cancer Diagnostics
- Market Opportunities in Liver Cancer Diagnostics Treatment
- Effects Of Future Therapies on Liver Cancer Diagnostics Treatment.
Liver Cancer Diagnostics Treatment Market Report Key Strengths
- 15 Years Liver Cancer Diagnostics Market Forecast
- Global Coverage
- Liver Cancer Diagnostics Epidemiology Segmentation
- Key Cross Competition
Liver Cancer Diagnostics Treatment Market Report Assessment
- Present Practices in the Liver Cancer Diagnostics Treatment Market
- Review of Investigational Liver Cancer Diagnostics Drugs
- Attractiveness of the Liver Cancer Diagnostics Drug Market
- Liver Cancer Diagnostics Market Drivers
- Liver Cancer Diagnostics Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the liver cancer diagnostics market based on the following segments:
Global Liver Cancer Diagnostics Market, By Test Type
- Laboratory Tests
- Imaging
- Endoscopy
- Biopsy
Global Liver Cancer Diagnostics Market, By End Use
- Hospitals & Diagnostic Laboratories
- Pharmaceutical & CRO Laboratories
Global Liver Cancer Diagnostics Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa