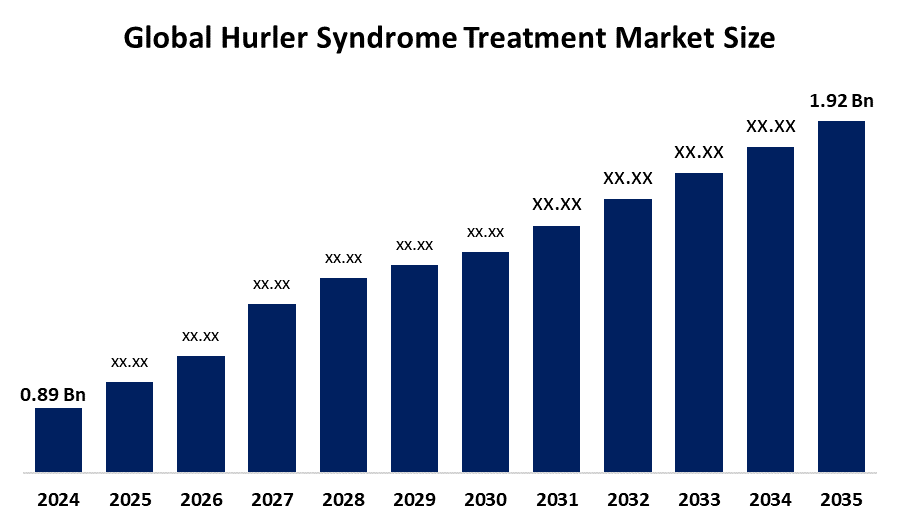
- As per Spherical Insights & Consulting, The Global Hurler Syndrome Treatment Market Size is expected to Grow from USD 0.89 Billion in 2024 to USD 1.92 Billion by 2035, at a CAGR of 7.24% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Hurler Syndrome Treatment Market Companies such as Sanofi, BioMarin Pharmaceutical, Orchard Therapeutics, Regenxbio, Esteve, JCR Pharmaceuticals, Green Cross Corp, Ultragenyx Pharmaceutical, Takeda Pharmaceutical, Sarepta Therapeutics, Abeona Therapeutics, Denali Therapeutics, Rocket Pharmaceuticals, Amicus Therapeutics, and Others.
Hurler Syndrome Treatment Market: Understanding and Treatment Algorithm:
The Hurler Syndrome Treatment Market encompasses therapies designed to manage Mucopolysaccharidosis Type I, including enzyme replacement therapy, stem cell transplantation, and supportive care. It focuses on improving patient outcomes by addressing systemic and neurological symptoms, driven by advances in medical research and growing awareness of rare genetic disorders globally.
Hurler Syndrome Treatment Diagnosis:
The Hurler Syndrome Diagnosis Market involves tools and methods used to identify Mucopolysaccharidosis Type I early, such as genetic testing, enzyme assays, and newborn screening. Accurate and timely diagnosis is critical for effective treatment, enabling early intervention and better management of this rare, progressive genetic disorder.
Hurler Syndrome Treatment:
The Hurler Syndrome Treatment Market includes therapies like enzyme replacement, hematopoietic stem cell transplantation, and supportive care aimed at managing symptoms and slowing disease progression. It focuses on improving patients’ quality of life by addressing both systemic and neurological effects of this rare genetic disorder through innovative medical approaches.
Hurler Syndrome Treatment Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Hurler Syndrome Treatment, Gender-specific Diagnosed Incidence of Hurler Syndrome Treatment, Type-specific Diagnosed Incidence of Hurler Syndrome Treatment, Age-specific Diagnosed Incidence of Hurler Syndrome Treatment, Diagnosed Incident Population based on Primary Site of Hurler Syndrome Treatment, and Diagnosed Incident Population based on Histologic Classification of Hurler Syndrome Treatment Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of Hurler syndrome treatment epidemiology in major markets worldwide.
Country-Wise Hurler Syndrome Treatment Multiforme Epidemiology
- The epidemiology segment provides Hurler Syndrome Treatment prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom) Asia-Pacific (including Japan) Latin America, the Middle East, and Africa.
Hurler Syndrome Treatment: Recent Developments:
- In July 2025, The HURCULES trial evaluating OTL-203, a one-time hematopoietic stem cell (HSC) gene therapy for Hurler syndrome, has completed patient enrollment nearly a year ahead of schedule. The company anticipates conducting the two-year primary outcome analysis following treatment.
Hurler Syndrome Treatment Marketed Drugs:
Aldurazyme: Sanofi and BioMarin Pharmaceutical
Aldurazyme (laronidase) is a recombinant form of the human α-L-iduronidase enzyme, indicated for Enzyme Replacement Therapy (ERT) in patients with Hurler Syndrome (MPS I). Approved by the FDA for treating non-neurological symptoms of MPS I, Aldurazyme aids in reducing glycosaminoglycan (GAG) buildup in body tissues, thereby enhancing respiratory function, physical mobility, and organ size. However, it is unable to cross the blood-brain barrier and does not address central nervous system involvement.
• Elaprase: Takeda Pharmaceutical
Elaprase (idursulfase) is an FDA-approved enzyme replacement therapy for MPS II (Hunter Syndrome). Although not indicated for Hurler Syndrome, it reflects progress in the treatment of mucopolysaccharidoses by improving mobility and respiratory function in affected patients.
• Mepsevii: Ultragenyx Pharmaceutical
Mepsevii (vestronidase alfa) is approved for the treatment of MPS VII, another subtype of mucopolysaccharidosis. This ERT has demonstrated the ability to reduce GAG accumulation and enhance endurance and physical capability, highlighting continued advancements in the management of lysosomal storage disorders.
Hurler Syndrome Treatment: Emerging Therapies
- OTL 203: OTL 203 is a late-stage investigational hematopoietic stem cell (HSC) gene therapy being developed for the treatment of Hurler Syndrome. Designed as a one-time therapy, it modifies the patient's own stem cells to enable continuous production of the deficient enzyme, potentially addressing both neurological and systemic manifestations more effectively than existing enzyme replacement therapies.
- JR 171: Currently in early clinical development, JR 171 is an innovative enzyme replacement therapy engineered to cross the blood-brain barrier. It is intended to treat both central nervous system and systemic symptoms of Hurler Syndrome, targeting neurological impairments that are not addressed by current therapies.
- RGX 111: RGX 111 is an investigational gene therapy developed by REGENXBIO, intended to deliver a functional IDUA gene directly to brain cells via an adeno-associated virus (AAV) vector. It is specifically designed to address the neurological manifestations of Hurler Syndrome and is currently undergoing early-stage clinical evaluation.
- ISP 001: ISP 001 is an autologous B-cell gene therapy currently in early-phase clinical trials, designed to enable continuous enzyme production by genetically modifying a patient’s B cells to express functional α-L-iduronidase. This therapeutic approach has the potential to minimize or eliminate the need for ongoing enzyme replacement infusions.
Hurler Syndrome Treatment Market Outlook
- The Hurler Syndrome treatment market involves therapies and interventions for Mucopolysaccharidosis Type I (MPS I), focusing on enzyme replacement, stem cell transplantation, and supportive care to manage symptoms and improve patient quality of life globally.
- Rising prevalence of Hurler Syndrome, advancements in enzyme replacement and gene therapies, growing awareness of rare diseases, increasing newborn screening, and enhanced healthcare infrastructure collectively drive demand in this specialized treatment market worldwide.
- Expanding gene therapy pipelines, emerging blood-brain barrier-crossing treatments, increasing adoption in developing regions, and collaborations between biotech firms and governments present significant growth opportunities in Hurler Syndrome treatment development and commercialization.
- Many governments have introduced rare disease policies, orphan drug incentives, newborn screening programs, and funding for genetic research, which collectively support faster diagnosis, improved access, and accelerated development of Hurler Syndrome therapies.
- High treatment costs and limited patient populations restrict widespread therapy adoption and affordability globally.
- The market is projected to grow robustly due to increasing investment in innovative gene and enzyme therapies targeting both systemic and neurological symptoms.
Hurler Syndrome Treatment Market Segmentation
By Treatment Type:
- Enzyme Replacement Therapy
- Hematopoietic Stem Cell Transplantation
- Supportive Care
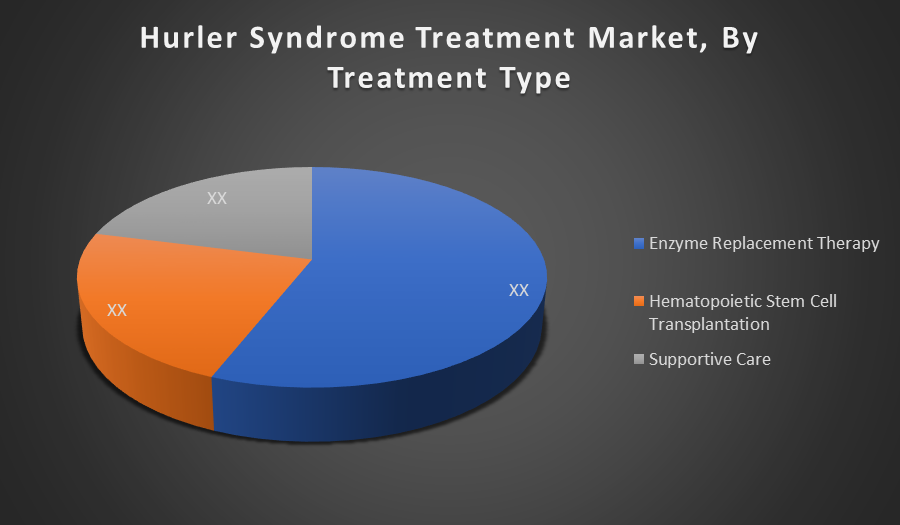
Enzyme Replacement Therapy holds the largest share in the Hurler Syndrome treatment market. It is the primary and most widely accepted treatment due to its ability to significantly improve clinical outcomes, reduce non-neurological symptoms, and enhance quality of life. Regulatory approvals and consistent advancements support its dominance.
By Patient Age Group:
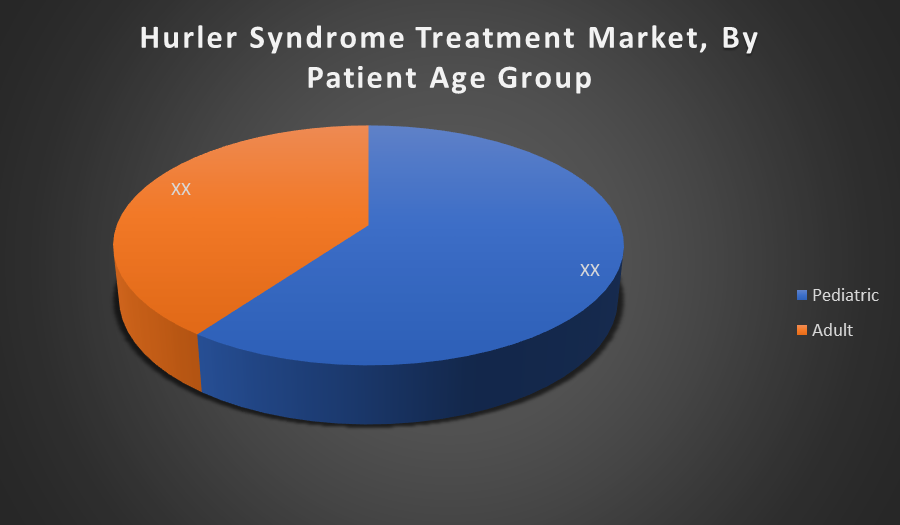
The Pediatric segment dominates the market as Hurler Syndrome typically manifests in early childhood. Early diagnosis and intervention are crucial for improving survival and quality of life. Hence, most treatment demand, including ERT and stem cell transplants, is concentrated in this age group, driving market share.
Regional Segment Analysis of the Hurler Syndrome Treatment Market
North America commands the largest share of the Hurler Syndrome treatment market, driven by its advanced healthcare infrastructure, strong presence of orphan drug programs, and widespread availability of approved therapies like Aldurazyme. The U.S. benefits from supportive FDA designations, comprehensive insurance coverage, and substantial research and development (R&D) investments. These factors create a highly conducive environment for both ERT and HSCT uptake, solidifying North America’s dominance in this segment
The Asia Pacific region is currently the fastest-growing market for Hurler Syndrome treatments, supported by rising healthcare spending, improved diagnostic capabilities, and enhanced awareness of rare diseases. Countries such as China, India, and Japan are expanding clinical trial initiatives and increasing access to advanced therapies; these developments, coupled with infrastructural improvement and government healthcare investments, fuel rapid market growth in APAC.
Hurler Syndrome Treatment Market Key Companies
- Sanofi
- BioMarin Pharmaceutical
- Orchard Therapeutics
- Regenxbio
- Esteve
- JCR Pharmaceuticals
- Green Cross Corp
- Ultragenyx Pharmaceutical
- Takeda Pharmaceutical
- Sarepta Therapeutics
- Abeona Therapeutics
- Denali Therapeutics
- Rocket Pharmaceuticals
- Amicus Therapeutics
- Others
Hurler Syndrome Treatment Therapeutics Market Report Scope
- The Hurler Syndrome Treatment therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Hurler Syndrome Treatment’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Hurler Syndrome Treatment therapies is provided, including an evaluation of new treatments expected to influence the current Hurler Syndrome Treatment market landscape.
- The report includes a detailed review of the Hurler Syndrome Treatment therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Hurler Syndrome Treatment Market Forecasting report offers valuable insights into trends shaping the global Hurler Syndrome Treatment market, helping to develop effective business strategies.
Hurler Syndrome Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Hurler Syndrome Treatment Therapeutic Approaches in Hurler Syndrome Treatment
- Review Of Drugs in Development for Hurler Syndrome Treatment
- Market, Growth, and Trends in Hurler Syndrome Treatment
- Market Opportunities in Hurler Syndrome Treatment
- Effects Of Future Therapies on Hurler Syndrome Treatment
Hurler Syndrome Treatment Market Report Key Strengths
- 15 Years Hurler Syndrome Treatment Market Forecast
- Global Coverage
- Hurler Syndrome Treatment Epidemiology Segmentation
- Key Cross Competition
Hurler Syndrome Treatment Market Report Assessment
- Present Practices in the Hurler Syndrome Treatment Market
- Review of Investigational Hurler Syndrome Treatment Drugs
- Attractiveness of the Hurler Syndrome Treatment Drug Market
- Hurler Syndrome Treatment Market Drivers
- Hurler Syndrome Treatment Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the hurler syndrome treatment market based on the below-mentioned segments:
Global Hurler Syndrome Treatment Market, By Treatment Type
- Enzyme Replacement Therapy
- Hematopoietic Stem Cell Transplantation
- Supportive Care
Global Hurler Syndrome Treatment Market, By Patient Age Group
Global Hurler Syndrome Treatment Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa






