DiGeorge Syndrome Treatment Market: Understanding and Treatment Algorithm:
DiGeorge syndrome is a rare genetic disorder caused by a deletion on chromosome 22 (22q11.2). It affects multiple body systems, leading to heart defects, immune deficiencies, low calcium levels, and developmental delays. Symptoms vary widely, and early diagnosis is key to managing complications through supportive therapies and specialized care.
DiGeorge Syndrome Diagnosis:
Diagnosis of DiGeorge syndrome involves a combination of clinical evaluation and genetic testing. Doctors assess physical symptoms such as heart defects and immune issues, followed by a fluorescence in situ hybridization (FISH) or microarray test to detect the 22q11.2 deletion. Early and accurate diagnosis helps guide appropriate treatment and management.
DiGeorge Syndrome Treatment:
Treatment of DiGeorge syndrome focuses on managing symptoms through a multidisciplinary approach. It includes calcium and vitamin D supplements for low calcium, immunotherapy for immune deficiencies, and surgery for heart defects. Hormone replacements and supportive therapies improve quality of life, while ongoing monitoring addresses developmental and psychiatric challenges.
DiGeorge Syndrome Drug Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of DiGeorge Syndrome Drug, Gender-specific Diagnosed Incidence of DiGeorge Syndrome Drug, Type-specific Diagnosed Incidence of DiGeorge Syndrome Drug, Age specific Diagnosed Incidence of DiGeorge Syndrome Drug, Diagnosed Incident Population based on Primary Site of DiGeorge Syndrome Drug, and Diagnosed Incident Population based on Histologic Classification of DiGeorge Syndrome Drug Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of DiGeorge syndrome drug epidemiology in major markets worldwide.
Country Wise DiGeorge Syndrome Drug Multiforme Epidemiology
- The epidemiology segment provides DiGeorge Syndrome Drug prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia Pacific (including Japan), Latin America, the Middle East, and Africa.
DiGeorge Syndrome Drug Marketed Drugs:
Rethymic (RVT 802) is a one time allogeneic cultured thymus tissue implant used to treat pediatric patients with congenital athymia, a severe form of DiGeorge syndrome. It restores immune function by enabling T cell development. Rethymic is FDA approved and the first treatment specifically indicated for complete DiGeorge anomaly.
- Calcitriol: Various Manufacturers
Calcitriol is a synthetic vitamin D analog used to manage hypocalcemia in DiGeorge syndrome by promoting calcium absorption. It helps compensate for parathyroid hormone deficiency and is often used in combination with calcium supplements. Widely marketed, it plays a critical role in endocrine symptom management.
- Calcium Carbonate: Various Manufacturers
Calcium carbonate is an oral calcium supplement used to correct hypocalcemia in DiGeorge syndrome. It supports bone health and neuromuscular function, especially in patients with parathyroid hormone deficiency. Available over the counter and by prescription, it remains a foundational therapy in symptom management.
DiGeorge Syndrome Drug: Emerging Therapies
- RVT802: This one time, tissue based regenerative therapy uses donor thymus tissue implanted to restore T cell development in congenital athymia, a severe immune deficiency common in complete DiGeorge syndrome. It holds Breakthrough Therapy and RMAT designations and is in late stage review following FDA feedback on manufacturing issues.
- NB 001: It is an oral, non stimulant activator of metabotropic glutamate receptors under Phase II trials. It aims to improve neuropsychiatric symptoms, such as anxiety, ADHD, and autism, often associated with 22q11.2 deletion syndrome. Early data show it's safe, well tolerated, and effective.
- Zygel: It is a transdermal cannabidiol gel in Phase II development for pediatric neuropsychiatric symptoms of 22q11.2 deletion syndrome. Its permeation enhanced delivery aims to manage anxiety and mood better than oral formulations. Initial trial data demonstrate statistically significant improvements in multiple behavioural scales.
- Thymus Transplantation: A pioneering cellular therapy where donor thymus tissue is transplanted to restore immune function in athymic infants. Though technically complex, it's the only long established curative option for complete DiGeorge anomaly.
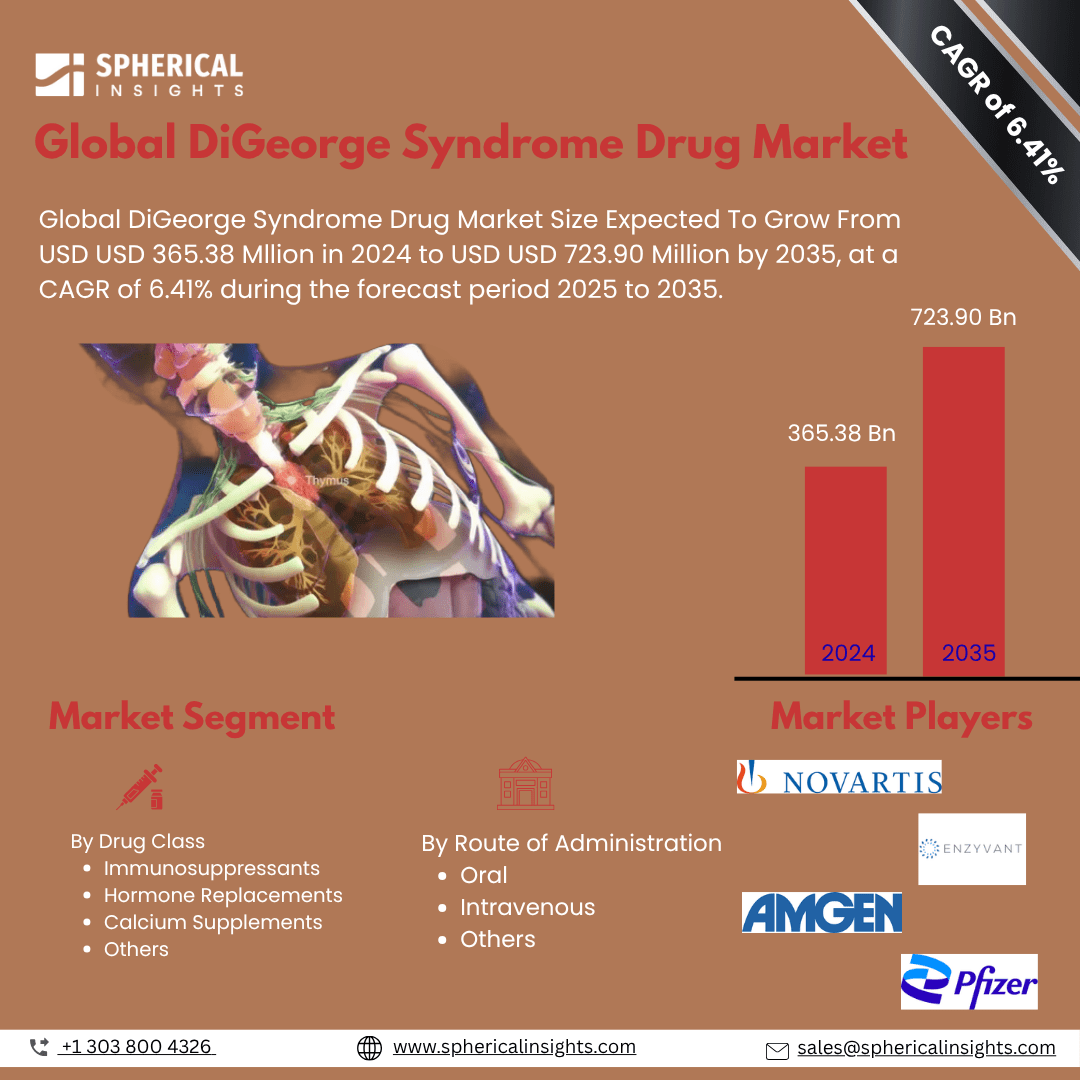
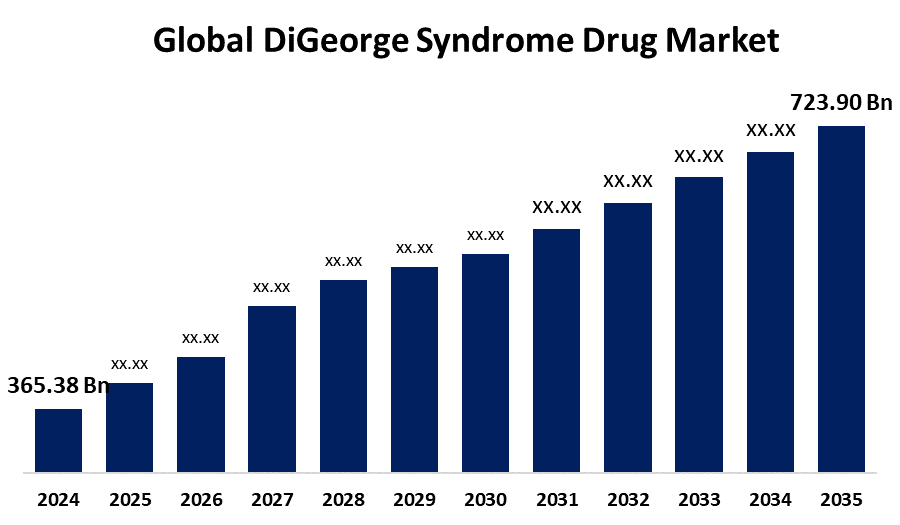
DiGeorge Syndrome Drug Market Outlook
- The DiGeorge Syndrome Drug Market comprises therapeutic products used to manage symptoms of DiGeorge syndrome, a rare genetic disorder affecting the immune system, heart, and endocrine functions. Treatments include immunosuppressants, hormone replacements, and calcium supplements tailored to support organ development and physiological stability in affected individuals.
- Key drivers include rising awareness of rare genetic disorders, increasing diagnosis rates, advancements in genomic research, and improved healthcare infrastructure. Enhanced availability of specialized treatments and government support for orphan drug development are accelerating demand for targeted therapies addressing the diverse manifestations of DiGeorge syndrome.
- Emerging markets with underdiagnosed populations offer vast potential for expansion. Growth in genetic testing, telemedicine, and personalized medicine is opening doors for early intervention and tailored therapies. Additionally, pharmaceutical investment in rare disease drug pipelines is creating new opportunities for innovative treatment development and commercialization.
- Governments are supporting DiGeorge syndrome management through rare disease policies, funding for genetic research, and incentives for orphan drug development. Programs promoting early screening, newborn testing, and global collaborations are also enhancing awareness, access, and treatment outcomes for affected populations.
- Limited disease awareness and delayed diagnosis remain major challenges restricting timely and effective treatment delivery.
- Projected growth is driven by rising diagnostic capabilities and increasing demand for specialized, targeted therapies.
DiGeorge Syndrome Drug Market Segmentation
By Drug Class:
- Immunosuppressants
- Hormone Replacements
- Calcium Supplements
- Others
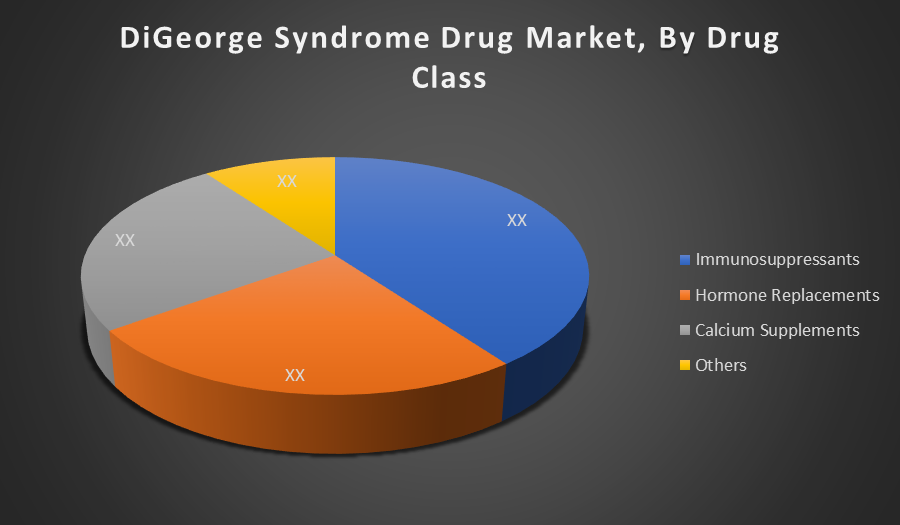
Immunosuppressants dominate the DiGeorge syndrome drug market due to the condition’s strong association with thymic hypoplasia and immune deficiency. These drugs help manage autoimmune disorders and prevent infections, making them essential in patient care. Their long term, regular use ensures higher demand and market share compared to other supportive drug classes.
By Route of Administration:
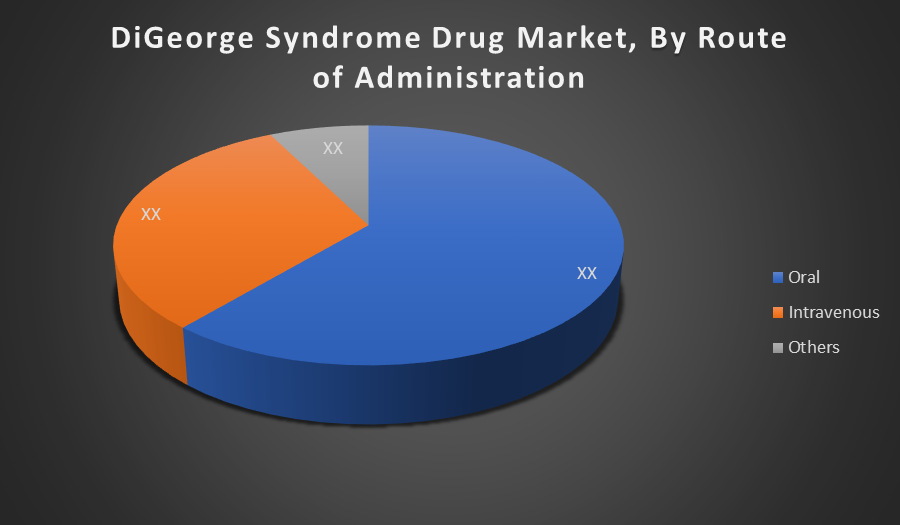
Oral administration holds the largest share in the DiGeorge syndrome drug market because it is the most convenient, non invasive, and cost effective method. Many treatments, including calcium supplements and hormone replacements, are formulated for oral intake. Patient compliance is higher with oral drugs, driving their preference and widespread market adoption.
Regional Segment Analysis of the DiGeorge Syndrome Drug Market
North America leads the DiGeorge syndrome drug market due to high disease awareness, advanced healthcare infrastructure, and significant investment in rare disease research. The region benefits from a strong pharmaceutical presence, active clinical trials, and supportive regulatory pathways. Robust diagnostic capabilities and favourable reimbursement policies also contribute to its dominance in the global market for DiGeorge syndrome treatments.
Asia Pacific is the fastest growing region in the DiGeorge syndrome drug market, driven by expanding healthcare infrastructure and increasing awareness. Rising investment in biotechnology and improvements in genetic diagnostics are fueling demand for treatment options. Countries across this region are focusing on rare disease policies and enhanced accessibility, making it an emerging hotspot for growth and innovation in DiGeorge syndrome therapeutics.
DiGeorge Syndrome Drug Market Key Companies
- Enzyvant
- Pfizer
- Novartis
- Merck & Co.
- Amgen
- Roche
- Johnson & Johnson
- Sanofi
- GSK
- Bayer
- Bristol Myers Squibb
- Takeda
- AbbVie
- Eli Lilly
- Biogen
- Others
DiGeorge Syndrome Drug Therapeutics Market Report Scope
- The DiGeorge Syndrome Drug Therapeutics Market Report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into DiGeorge Syndrome Drug’s epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging DiGeorge Syndrome Drug therapies is provided, including an evaluation of new treatments expected to influence the current DiGeorge Syndrome Drug treatment market landscape.
- The report includes a detailed review of the DiGeorge Syndrome Drug therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient Based DiGeorge Syndrome Drug Market Forecasting report offers valuable insights into trends shaping the global DiGeorge Syndrome Drug market, helping to develop effective business strategies.
DiGeorge Syndrome Drug Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- DiGeorge Syndrome Drug Therapeutic Approaches in DiGeorge Syndrome Drug
- Review Of Drugs in Development for DiGeorge Syndrome Drug
- Market, Growth, and Trends in DiGeorge Syndrome Drug
- Market Opportunities in DiGeorge Syndrome Drug Treatment
- Effects Of Future Therapies on DiGeorge Syndrome Drug Treatment.
DiGeorge Syndrome Drug Treatment Market Report Key Strengths
- 15 Years DiGeorge Syndrome Drug Market Forecast
- Global Coverage
- DiGeorge Syndrome Drug Epidemiology Segmentation
- Key Cross Competition
DiGeorge Syndrome Drug Treatment Market Report Assessment
- Present Practices in the DiGeorge Syndrome Drug Treatment Market
- Review of Investigational DiGeorge Syndrome Drug Drugs
- Attractiveness of the DiGeorge Syndrome Drug Drug Market
- DiGeorge Syndrome Drug Market Drivers
- DiGeorge Syndrome Drug Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the DiGeorge syndrome drug market based on the below mentioned segments:
Global DiGeorge Syndrome Drug Market, By Drug Class
- Immunosuppressants
- Hormone Replacements
- Calcium Supplements
- Others
Global DiGeorge Syndrome Drug Market, By Route of Administration
Global DiGeorge Syndrome Drug Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa