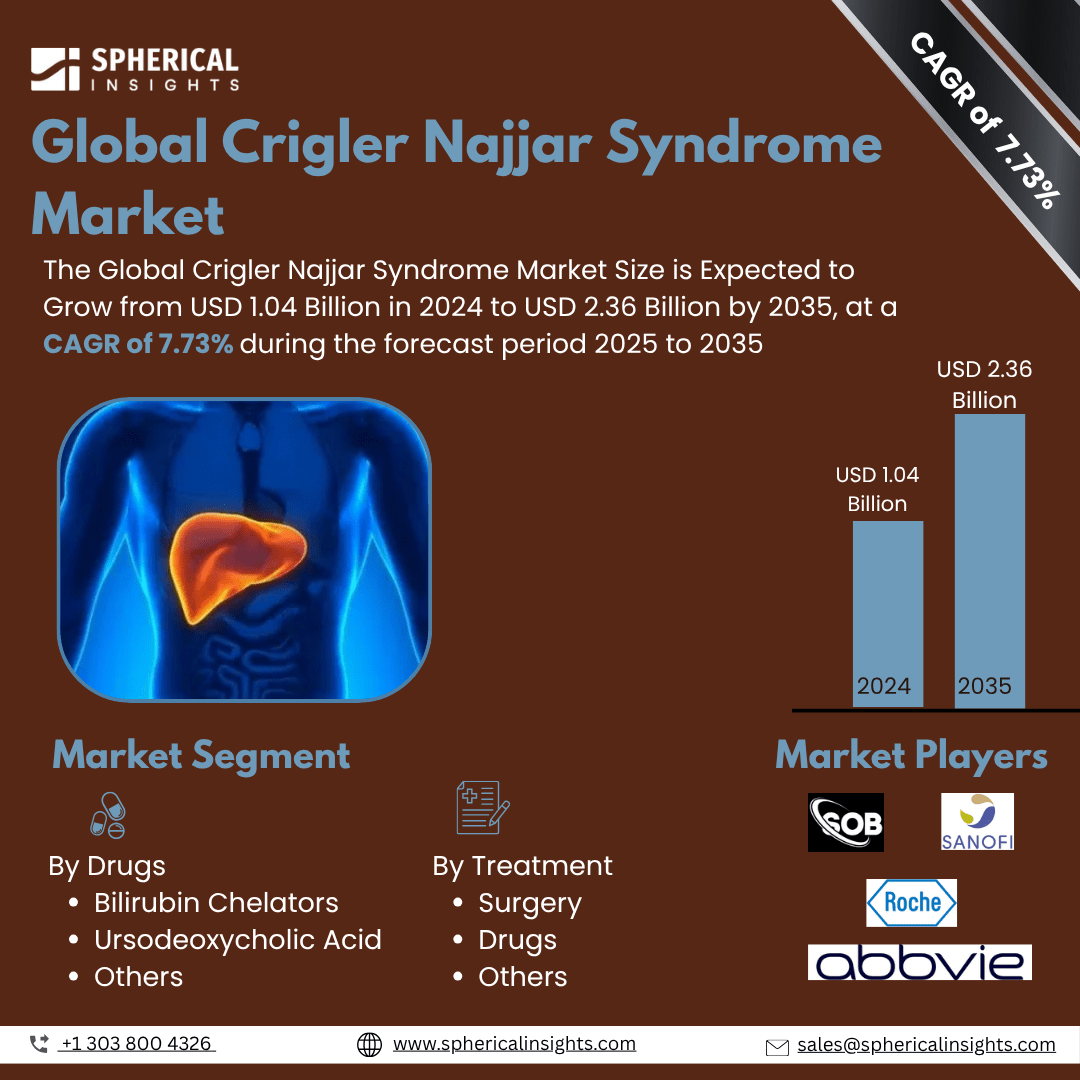
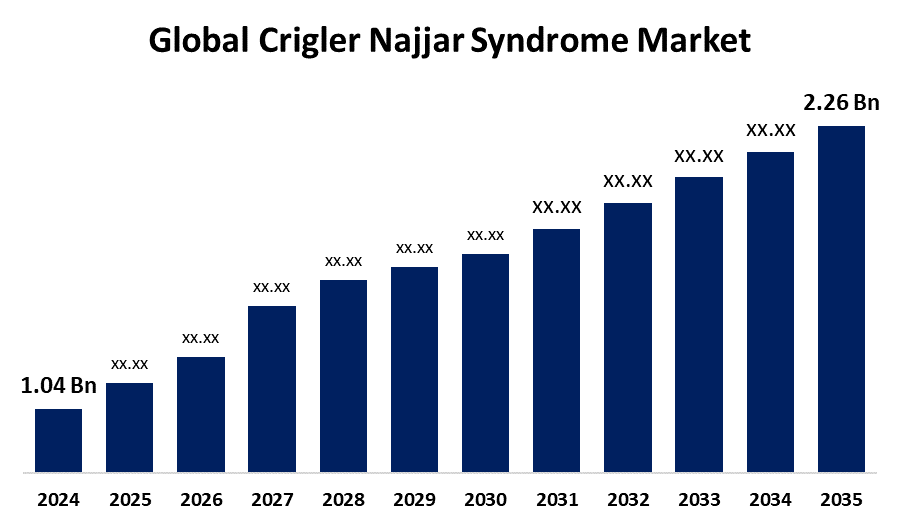
- As per Spherical Insights & Consulting, The Global Crigler Najjar Syndrome Market Size is Expected to Grow from USD 1.04 Billion in 2024 to USD 2.36 Billion by 2035, at a CAGR of 7.73% during the forecast period 2025 to 2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Crigler Najjar Syndrome Market Companies such as Sobi, BioMarin Pharmaceuticals, Ultragenyx Pharmaceutical, Amicus Therapeutics, Sanofi, Novartis, Takeda Pharmaceutical, Pfizer, Roche, Johnson & Johnson, Merck, AbbVie, Bristol-Myers Squibb, Vertex Pharmaceuticals, CSL Behring, and Others.
Crigler Najjar Syndrome Treatment Market: Understanding and Treatment Algorithm:
Crigler Najjar Syndrome is a rare genetic disorder causing severe jaundice due to the liver’s inability to process bilirubin. This leads to toxic bilirubin buildup, risking brain damage. It primarily affects newborns and is classified into two types, Type 1 (severe) and Type 2 (milder), based on the severity of the enzyme deficiency.
Crigler Najjar Syndrome Diagnosis:
Diagnosis involves measuring high levels of unconjugated bilirubin in blood tests and ruling out other liver diseases. Genetic testing confirms mutations in the UGT1A1 gene. Additional assessments include liver function tests, phototherapy response, and family history evaluation to distinguish between Type 1 and Type 2 Crigler Najjar Syndrome.
Crigler Najjar Syndrome Treatment:
Treatment focuses on reducing bilirubin levels to prevent complications. Phototherapy is used to break down bilirubin, especially in infants. Drugs like phenobarbital help increase enzyme activity in Type 2. Severe cases may require liver transplantation or emerging gene therapies aimed at correcting the enzyme deficiency.
Crigler Najjar Syndrome Epidemiology:
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Crigler Najjar Syndrome, Gender specific Diagnosed Incidence of Crigler Najjar Syndrome, Type specific Diagnosed Incidence of Crigler Najjar Syndrome, Age specific Diagnosed Incidence of Crigler Najjar Syndrome, Diagnosed Incident Population based on Primary Site of Crigler Najjar Syndrome, and Diagnosed Incident Population based on Histologic Classification of Crigler Najjar Syndrome Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights:
This section offers a global overview of Crigler Najjar Syndrome epidemiology in major markets worldwide.
Country Wise Crigler Najjar Syndrome Multiforme Epidemiology:
- The epidemiology segment provides Crigler Najjar Syndrome prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Crigler Najjar Syndrome: Recent Developments:
- In December 2024, Genethon and Hansa Biopharma announced the initiation of a Phase 2 clinical trial to evaluate imlifidase as a pre treatment for Genethon’s gene therapy GNT 0003 in patients with severe Crigler Najjar syndrome who have pre existing anti AAV8 antibodies. This aims to overcome antibody related treatment barriers.
Crigler Najjar Syndrome Marketed Drugs:
• Phenobarbital: Various Manufacturers
Phenobarbital is a barbiturate that induces the activity of the UGT1A1 enzyme, helping reduce bilirubin levels in patients with Crigler Najjar Syndrome Type 2. It is commonly used as a first-line drug to manage mild to moderate unconjugated hyperbilirubinemia, but is ineffective in Type 1 cases.
• Ursodeoxycholic Acid (UDCA): Various Manufacturers
Ursodeoxycholic Acid is a bile acid used to improve bile flow and reduce liver toxicity. Although not a direct treatment for Crigler Najjar Syndrome, it is sometimes used as supportive therapy to protect liver function and aid in bilirubin elimination in affected patients.
Crigler Najjar Syndrome: Emerging Therapies:
- GNT 0003: It is an AAV8 based gene therapy designed to deliver a functional UGT1A1 gene to liver cells, aiming to restore bilirubin metabolism in patients with Crigler Najjar Syndrome Type 1. It is currently in late stage clinical trials showing promise in reducing toxic bilirubin levels.
- ATB 101: It is an enzyme replacement therapy that uses a modified UGT1A1 enzyme fused with a stabilizing domain to increase bilirubin clearance. This investigational therapy is in early clinical development and targets both Type 1 and Type 2 patients to reduce long term complications.
Crigler Najjar Syndrome Market Outlook:
- The Crigler Najjar Syndrome market includes all therapies, diagnostics, and treatment options aimed at managing this rare genetic disorder characterized by high bilirubin levels. It covers drugs, gene therapies, and surgical interventions used globally to improve patient outcomes and quality of life.
- Rising awareness of rare genetic diseases, advancements in gene therapy, and increasing diagnosis rates drive market growth. Additionally, unmet medical needs for effective treatments and growing investments by pharmaceutical companies accelerate research and product development in Crigler Najjar Syndrome.
- Emerging gene therapies, enzyme replacement treatments, and personalized medicine offer significant growth potential. Expanding newborn screening programs and collaborations between biotech firms and research institutes create opportunities to develop innovative, targeted treatments for this underserved patient population.
- Governments worldwide support rare disease research through funding, orphan drug designations, and regulatory incentives. These initiatives aim to accelerate drug approvals and improve access to treatments for Crigler Najjar Syndrome patients, fostering innovation and encouraging pharmaceutical investment.
- Limited patient population and high treatment costs restrict widespread adoption of novel therapies.
- The market is projected to grow significantly due to advances in gene therapy technologies and increasing global diagnosis rates.
Crigler Najjar Syndrome Market Segmentation:
By Drugs:
- Bilirubin Chelators
- Ursodeoxycholic Acid
- Others
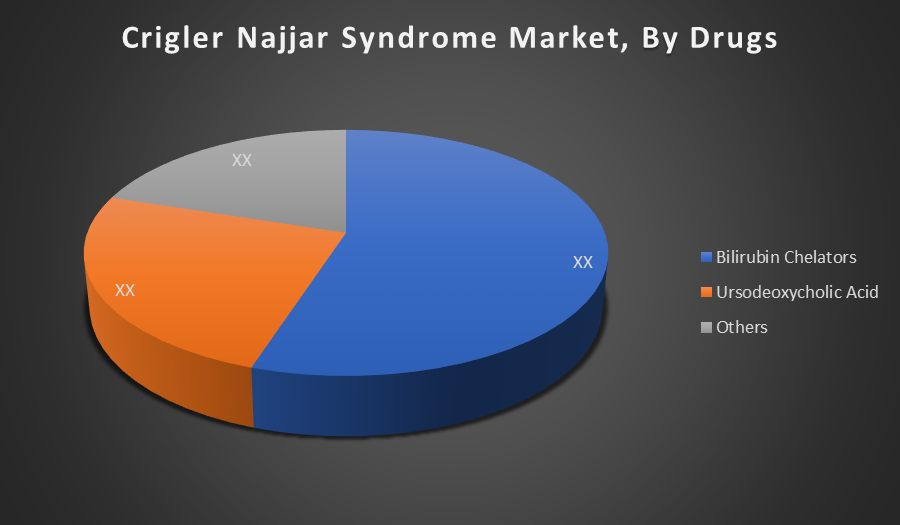
The Bilirubin Chelators segment holds the largest share because these drugs directly target and reduce toxic bilirubin levels, which is the primary cause of Crigler Najjar Syndrome complications. Their effectiveness and central role in managing symptoms drive higher market demand compared to Ursodeoxycholic Acid and other drugs.
By Treatment:
The Drugs segment dominates over surgery and others due to its non-invasive nature, lower risks, and wider applicability across patient types. Surgical options like liver transplantation are limited by donor availability and high costs, making drug treatment the preferred and more accessible choice.
Regional Segment Analysis of the Crigler Najjar Syndrome Market:
North America holds the largest share in the Crigler Najjar Syndrome market, driven by advanced healthcare infrastructure, high awareness of rare diseases, and strong pharmaceutical presence. The region benefits from significant investments in gene therapy research and well established regulatory frameworks that support orphan drug approvals. Additionally, widespread access to diagnostic technologies and patient support programs enhances early diagnosis and treatment adoption, maintaining North America’s leading market position.
The Asia Pacific region is the fastest growing market for Crigler Najjar Syndrome due to increasing healthcare expenditure, growing awareness of rare genetic disorders, and expanding diagnostic capabilities. Rapid improvements in healthcare infrastructure and rising government initiatives to support rare disease treatment are fueling market growth. Moreover, collaborations between local biotech firms and global pharmaceutical companies accelerate the introduction of advanced therapies, driving the region’s dynamic market expansion.
Crigler Najjar Syndrome Market Key Companies:
- Sobi
- BioMarin Pharmaceuticals
- Ultragenyx Pharmaceutical
- Amicus Therapeutics
- Sanofi
- Novartis
- Takeda Pharmaceutical
- Pfizer
- Roche
- Johnson & Johnson
- Merck
- AbbVie
- Bristol Myers Squibb
- Vertex Pharmaceuticals
- CSL Behring
- Others
Market Segment:
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Crigler Najjar Syndrome market based on the following segments:
Global Crigler Najjar Syndrome Market, By Drugs
- Bilirubin Chelators
- Ursodeoxycholic Acid
- Others
Global Crigler Najjar Syndrome Market, By Treatment
Global Crigler Najjar Syndrome Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa