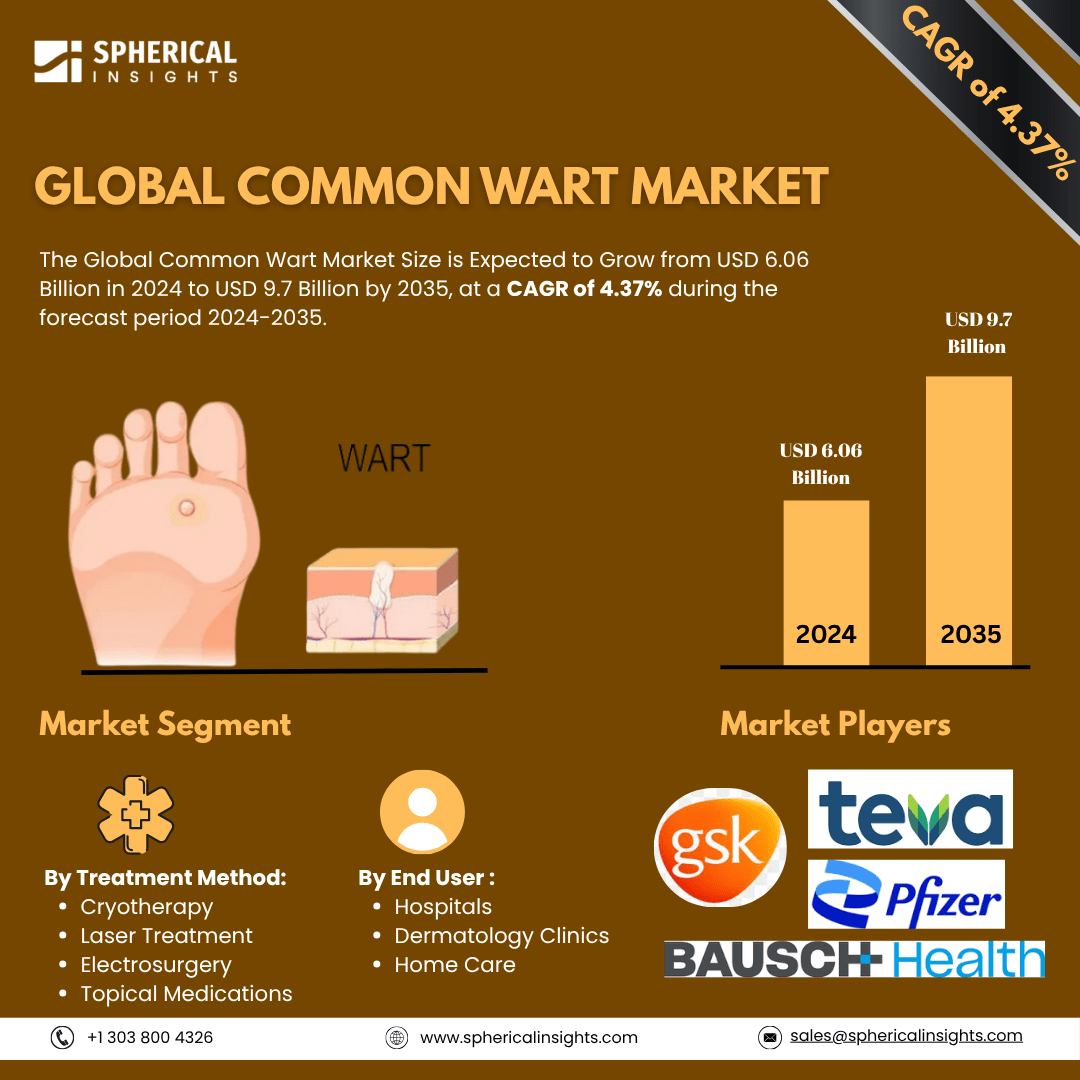
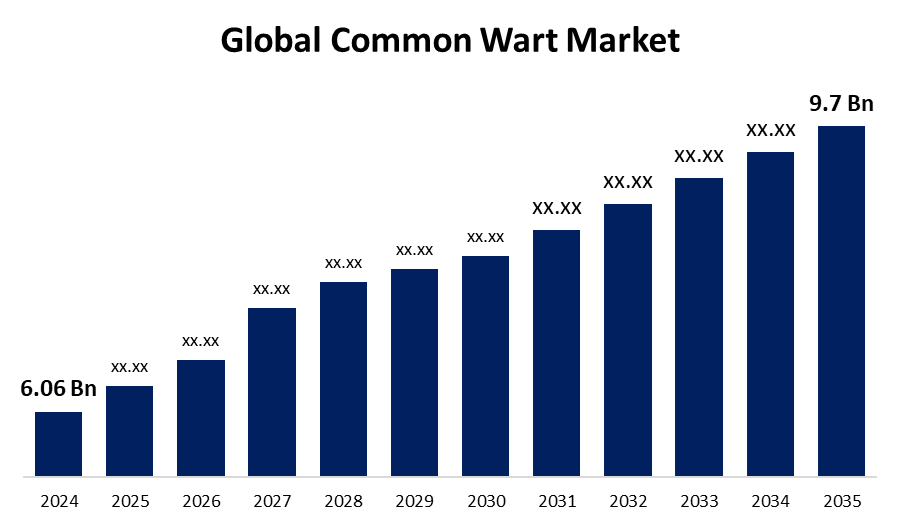
- As per Spherical Insights & Consulting, The Global Common Wart Market Size is Expected to Grow from USD 6.06 Billion in 2024 to USD 9.7 Billion by 2035, at a CAGR of 4.37% during the forecast period 2025-2035, owing to the launch of new therapies in the market and the rise in the number of cases.
- Warts can occur at any age. Although rare in infancy and early childhood, prevalence increases among school-aged children and peaks at 12 to 16 years.
- The male-to-female ratio is approximately equal.
- The leading Common Wart Market Companies such as GlaxoSmithKline, Merck & Co., Bausch Health, Teva Pharmaceuticals, Pfizer, Meda Pharmaceuticals, Cipla, Mylan, Perrigo, Taro Pharmaceutical, Novartis, Sanofi, AbbVie, Lytix Biopharma, Verrica Pharmaceuticals, and others.
Common Wart Treatment Market: Understanding and Treatment Algorithm:
Common warts are small, rough skin growths caused by human papillomavirus (HPV) infection. They typically appear on hands, fingers, and knees and are contagious through direct contact. Though harmless, warts can be unsightly and sometimes painful. Treatments include topical medications, cryotherapy, and minor surgical removal to eliminate the lesions effectively.
Common Wart Diagnosis:
Diagnosis is primarily clinical, based on the appearance of rough, raised skin lesions with a characteristic cauliflower-like surface. Doctors may use a dermatoscope for closer examination. In uncertain cases, a biopsy or HPV testing can confirm the diagnosis and rule out other skin conditions.
Common Wart Treatment
Treatment options include topical salicylic acid, cryotherapy (freezing with liquid nitrogen), laser therapy, and minor surgical removal. Immunotherapy and chemical peels may be used for stubborn warts. Treatment aims to eliminate the wart, prevent spread, and reduce recurrence, though warts can sometimes resolve spontaneously over time.
Common Wart Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Common Wart, Gender-specific Diagnosed Incidence of Common Wart, Type-specific Diagnosed Incidence of Common Wart, Age-specific Diagnosed Incidence of Common Wart, Diagnosed Incident Population based on Primary Site of Common Wart, and Diagnosed Incident Population based on Histologic Classification of Common Wart Tumour in the global market covering North America, Europe, Asia-Pacific, Latin America, the Middle East, and Africa from 2024 to 2035.
Principal Insights
This section offers a global overview of Common Wart epidemiology in major markets worldwide.
Country Wise- Common Wart Multiforme Epidemiology
- The epidemiology segment provides Common Wart prevalence data and findings across key regions worldwide, including North America, Europe (Germany, France, Italy, Spain, and the United Kingdom), Asia-Pacific (including Japan), Latin America, the Middle East, and Africa.
Common Wart Recent Developments:
- In January 2024, Verrica announced the outcome of a Type C meeting with the FDA, confirming alignment on the Phase 3 trial design for YCANTH in treating common warts. The decision followed promising Phase 2 results, supporting further development and validating the proposed clinical endpoints and overall study approach.
Common Wart Marketed Drugs:
- VP-102 (Cantharidin 0.7%): Verrica Pharmaceuticals
VP-102 is a topical drug-device combination containing cantharidin, a vesicant that causes controlled blistering of wart tissue, leading to immune-mediated clearance. It is being developed specifically for common warts and has completed Phase 2 trials, with Phase 3 trials planned.
- CANDIN (Candida antigen): Nielsen BioSciences
CANDIN is an intradermal immunotherapy derived from Candida albicans. It stimulates a systemic immune response, helping the body recognize and clear cutaneous warts. It is used off-label and is currently in Phase 3 trials for FDA approval in treating common warts.
- Imiquimod (Aldara): Meda AB (a Mylan company)
Imiquimod is a topical immune response modifier that activates toll-like receptor 7 (TLR7), enhancing innate and adaptive immunity. Though FDA-approved for genital warts, it is used off-label for recalcitrant common warts.
Common Wart: Emerging Therapies
- YCANTH (VP-102): YCANTH is a proprietary topical formulation of cantharidin in late-stage clinical development by Verrica Pharmaceuticals. It induces blistering to remove wart tissue and stimulate immune clearance, targeting patients with refractory common warts.
- CANDIN (Candida antigen): CANDIN is an intralesional immunotherapy derived from Candida albicans, currently in Phase 3 trials by Nielsen BioSciences. It activates systemic immune responses to promote the clearance of multiple and recalcitrant warts.
- SB206 (Nitric oxide releasing gel): SB206 is a topical gel developed by Novan Therapeutics that delivers controlled nitric oxide to wart tissue, enhancing local immune response and antiviral activity. It is in Phase 3 trials for common warts.
- Ciclopirox olamine: Being investigated as a topical antifungal agent with immunomodulatory properties, ciclopirox is showing promise in preclinical studies for treating recalcitrant common warts.
Common Wart Market Outlook
- The Common Wart Market encompasses the development, production, and sales of treatments and diagnostic products for common warts, benign skin growths caused by HPV. It includes topical therapies, cryotherapy devices, and other medical solutions aimed at effectively managing and removing warts worldwide.
- Key drivers of the common wart market include increasing prevalence of HPV infections, rising awareness of skin health, demand for effective and minimally invasive treatments, growing access to dermatological care, and technological advancements in therapeutic products and procedures globally.
- Market opportunities in the common wart segment include developing innovative, painless treatments, expanding access in emerging regions, leveraging teledermatology for remote diagnosis, increasing adoption of combination therapies, and growing investments in research for HPV-related skin conditions to improve patient outcomes.
- Government initiatives include HPV vaccination programs like India’s Cervavac, public awareness campaigns, and integration of HPV screening into healthcare services, aiming to reduce HPV infections and lower common wart prevalence globally.
- Challenges include HPV vaccine hesitancy, limited awareness, and inconsistent access to effective wart treatments, hindering widespread prevention and management of common warts globally.
- The market is expected to grow steadily due to increasing HPV awareness, expanding vaccination programs, and advances in non-invasive treatment options, driving higher adoption and improved patient outcomes.
Common Wart Market Segmentation
By Treatment Method:
- Cryotherapy
- Laser Treatment
- Electrosurgery
- Topical Medications
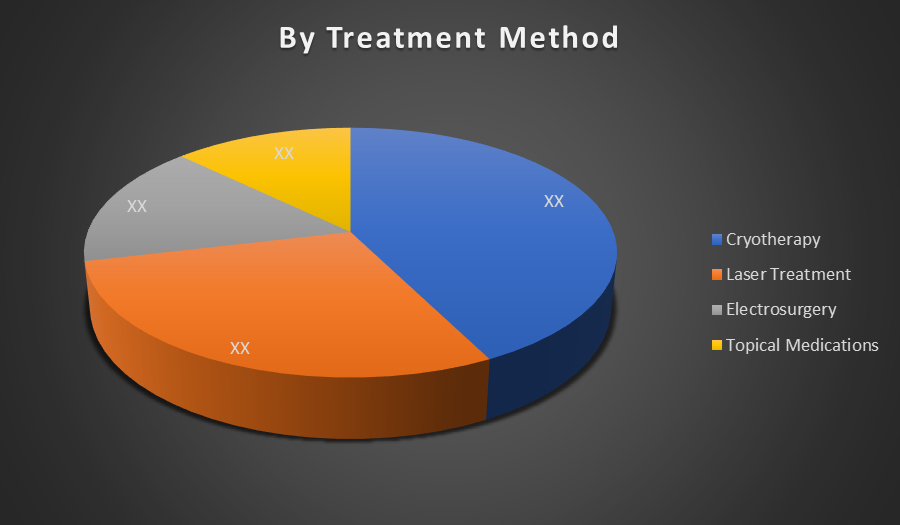
Cryotherapy dominated the global common wart market due to its high efficacy, rapid results, and widespread availability in clinical settings. It is a preferred first-line treatment by dermatologists, offering minimal invasiveness and low recurrence rates, making it more favourable than topical drugs or surgical options.
By End User:
- Hospitals
- Dermatology Clinics
- Home Care
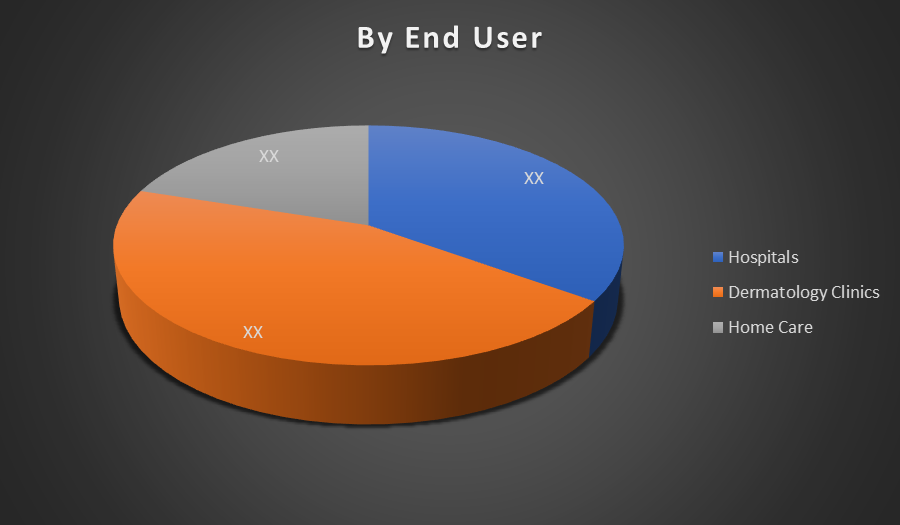
Dermatology clinics dominated the global common wart market due to specialized care, access to advanced treatments like cryotherapy and laser, and higher patient preference for expert-led procedures. These clinics balance efficiency, affordability, and quality care, making them more attractive than hospitals or home-based treatment options.
Regional Segment Analysis of the Common Wart Market
North America dominates the common warts market primarily due to its advanced healthcare infrastructure and high patient awareness. The region benefits from widespread access to dermatologists and specialists who can diagnose and treat warts effectively. Additionally, North America has a strong presence of leading pharmaceutical companies investing heavily in research and development of innovative wart treatments, including novel immunotherapies and topical agents. Well-established regulatory frameworks and favourable reimbursement policies also encourage the adoption of newer therapies. Furthermore, the availability of over-the-counter treatments and high consumer spending on healthcare products contribute to robust market growth.
The Asia Pacific region is projected to be the fastest-growing market for common wart treatments. This growth is driven by factors such as improving healthcare infrastructure, increasing disposable incomes, and heightened awareness of dermatological health, particularly in countries like China, India, and Japan. The region's expanding middle class and greater access to healthcare services contribute to the rising demand for effective wart treatments. Additionally, the Asia Pacific market benefits from a large patient pool, making it an attractive area for pharmaceutical companies to invest in and develop new therapies.
Common Wart Market Key Companies
- GlaxoSmithKline
- Merck & Co.
- Bausch Health
- Teva Pharmaceuticals
- Pfizer
- Meda Pharmaceuticals
- Cipla
- Mylan
- Perrigo
- Taro Pharmaceutical
- Novartis
- Sanofi
- AbbVie
- Lytix Biopharma
- Verrica Pharmaceuticals
- Others
Common Wart Therapeutics Market Report Scope
- The Common Wart therapeutics market report provides a detailed overview, covering its causes, symptoms, disease progression, and existing treatment options.
- Detailed insights into Common Warts’ epidemiology and therapeutic approaches are included.
- Additionally, a comprehensive review of existing and emerging Common Wart therapies is provided, including an evaluation of new treatments expected to influence the current Common Wart treatment market landscape.
- The report includes a detailed review of the Common Wart therapeutics market, both historical and forecasted, highlighting the global drug reach.
- The Patient-Based Common Wart Market Forecasting report offers valuable insights into trends shaping the global Common Wart market, helping to develop effective business strategies.
Common Wart Treatment Market Report Insights
- Forecasting Market Trends Based on Patient Data and Disease Rates
- Common Wart Therapeutic Approaches in Common Wart
- Review Of Drugs in Development for Common Wart
- Market, Growth, and Trends in Common Wart
- Market Opportunities in Common Wart Treatment
- Effects Of Future Therapies on Common Wart Treatment.
Common Wart Treatment Market Report Key Strengths
- 15 Years Common Wart Market Forecast
- Global Coverage
- Common Wart Epidemiology Segmentation
- Key Cross Competition
Common Wart Treatment Market Report Assessment
- Present Practices in the Common Wart Treatment Market
- Review of Investigational Common Wart Drugs
- Attractiveness of the Common Wart Drug Market
- Common Wart Market Drivers
- Common Wart Market Barriers
- SWOT
- Attribute Analysis
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2025 to 2035. Spherical Insights has segmented the common wart market based on the below-mentioned segments:
Global Common Wart Market, By Treatment Method
- Cryotherapy
- Laser Treatment
- Electrosurgery
- Topical Medications
Global Common Wart Market, By End User
- Hospitals
- Dermatology Clinics
- Home Care
Global Common Wart Market, By Regional Analysis
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa