
Top 50 Companies in Clinical Trials Market in the World in 2025: Market Research Report (2024–2035)
RELEASE DATE: Aug 2025 Author: Spherical InsightsRequest Free Sample Speak to Analyst
Description
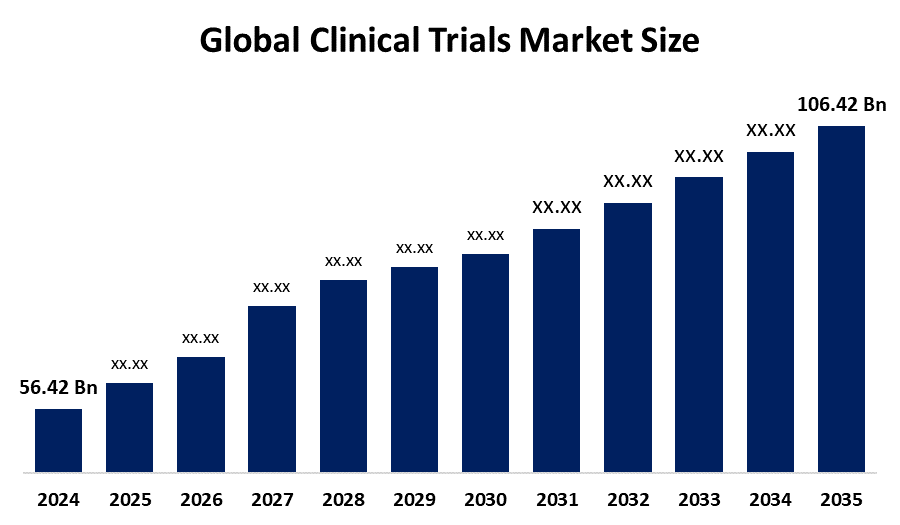
According to a research report published by Spherical Insights & Consulting, The Global Clinical Trials Market Size is projected to grow from USD 56.42 Billion in 2024 to USD 106.42 Billion by 2035, at a CAGR of 6.55 % during the forecast period 2025-2035. The clinical trials market holds future opportunities in decentralized trials, AI-driven patient recruitment, personalized medicine, global trial expansion, and real-world data integration, enhancing efficiency, inclusivity, and faster drug development.
Introduction
The clinical trials market refers to the global industry involved in the research and testing of new drugs, medical devices, and treatment protocols through human participation. These trials are essential for evaluating safety, efficacy, and regulatory approval before products reach the market. Several driving factors are fueling the growth of this market. The increasing prevalence of chronic diseases and the aging global population are creating a heightened demand for innovative therapies. Technological advancements such as AI, wearable monitoring devices, and decentralized trial platforms are enhancing trial efficiency and patient engagement. Additionally, rising investments in R&D by pharmaceutical companies and supportive government policies are further accelerating market expansion.
Navigate Future Markets with Confidence: Insights from Spherical Insights LLP
The insights presented in this blog are derived from comprehensive market research conducted by Spherical Insights LLP, a trusted advisory partner to leading global enterprises. Backed by in-depth data analysis, expert forecasting, and industry-specific intelligence, our reports empower decision-makers to identify strategic growth opportunities in fast-evolving sectors. Clients seeking detailed market segmentation, competitive landscapes, regional outlooks, and future investment trends will find immense value in the full report. By leveraging our research, businesses can make informed decisions, gain a competitive edge, and stay ahead in the transition toward sustainable and profitable solutions.
Unlock exclusive market insights - Download the Brochure now and dive deeper into the future of the clinical trials market.
Clinical Trials Market Size & Statistics
- The Market for Clinical Trials was estimated To be worth USD 56.42 Billion in 2024.
- The Market is Going to expand at a CAGR of 6.55 % between 2025 and 2035.
- The Global Clinical Trials Market Size is anticipated to reach USD 106.42 Billion by 2035.
- North America is expected to generate the highest demand during the forecast period in the Clinical Trials market.
- Asia Pacific is expected to grow the fastest during the forecast period in the Clinical Trials market.
Regional growth and demand
Asia Pacific is expected to grow the fastest during the forecast period in the clinical trials market. The Asia Pacific region is anticipated to experience the highest growth throughout the forecast period. This is because of the easily accessible extensive patient population, enabling simpler recruitment for clinical trials. "Novotech," the most experienced and largest biotech CRO in Asia Pacific, has noticed an increase in inquiries from biotechnology sponsors for studies because of their rapid turnaround and high quality.
North America is expected to generate the highest demand during the forecast period in the clinical trials market. North America is leading the market with over 53.7% market share during the forecast period. This growth has been attributed to enhanced financing for research and development and a higher use of cutting-edge technology in clinical trials within this area. Additionally, North America's prominent standing in the clinical trials market stems from swift growth in the biologic and biosimilar sectors, along with heightened investments in advancing clinical research. Moreover, North America possesses the largest pharmaceutical market globally, featuring many major international pharmaceutical and medical device companies based there, such as Pfizer, Inc., AbbVie, Inc., Abbott Laboratories, and Johnson & Johnson. Additionally, favorable government support for clinical research in the US market is anticipated to drive demand for the clinical trials market in the area throughout the projected period.
Empower your strategic planning:
Stay informed with the latest industry insights and market trends to identify new opportunities and drive growth in the clinical trials market. To explore more in-depth trends, insights, and forecasts, please refer to our detailed report.
Top 5 Clinical Trial Solutions to Watch
1. Koneksa Health – Real-World Clinical Data Platform
Biometric devices, artificial intelligence (AI), advanced analytics, wearables, and cloud management technologies greatly enhance clinical trials. Gathering and examining precise clinical trial data is essential for pharmaceutical research and development (R&D) to identify and disseminate innovative therapies. For instance, transitioning conventional data gathering to digital platforms facilitates the clinical trial process and enhances patient compliance with the trial. Danish startup Koneksa Health develops Koneksa Compare, a platform designed to collect, track, and evaluate real-world data and outcomes generated by patients in clinical trials. The startup provides assistance for remote biometric devices that regularly record data, including beyond the restriction period. Koneksa’s electronic clinical outcome assessments (eCOAs) allow pharmaceutical R&D teams to enhance the trustworthiness of patient-reported information. The startup’s dashboards, alerts, and adaptable platform guarantee the proper utilization and adherence to clinical trials data.
2. Trials.ai – Clinical Trials Design Platform
Several significant obstacles that impede the success of clinical trials include adherence to intricate regulations and design specifications. Consequently, R&D teams are always pursuing the best design for a clinical study to guarantee that all parties involved, from patients to regulators, can effortlessly engage in a positive result. A properly structured clinical trial is also crucial to make certain that expenses stay within the allocated budget. This motivates startups to create technology-driven designs for clinical trials. Trials.ai is a startup located in the US that creates a smart protocol based on AI to enhance the design of clinical trials. The Trials.ai platform utilizes machine learning techniques on numerous documents to enhance clinical trial protocols. The Smart Protocol Builder of the startup allows for focused training and adjustment of clinical trial ontology (CTO), thus enhancing the study's accuracy. The final outcome is a clinical trial design focused on patients that maximizes time, expenses, and associated risks.
3. PHARMASEAL – Clinical Trials Management Platform
Once data is gathered and a suitable strategy for the trial is developed, clinical research organizations (CRO) must effectively oversee the complete study. Innovative healthcare and pharmaceutical technology startups aim to consolidate all pertinent clinical trial management data within a unified platform. This encompasses the integration of clinical trial management systems (CTMS) and the electronic trial master file (eTMF). UK-based clinical trial management company PHARMASEAL employs sophisticated automation to create and operate a comprehensive enterprise platform that enables biopharmaceutical and medical device firms to efficiently manage their clinical trials. The startup’s Engility trial management system is a cloud-based, scalable, and collaborative platform that integrates CTMS and eTMF. Engility’s integrated trial management consolidates data regarding the study, patients, agreements, payments, documentation, and monitoring to enhance the effectiveness and productivity of clinical trials.
4. ConsilX – Blockchain-Powered Patient Management
The last component of a successful clinical trial entails ongoing and dependable patient observation, involvement, and administration. This involves securing patient consent and enrolling them in the trial, interacting with patients during the study, and guaranteeing the availability of the necessary tools or devices for the clinical trial. Moreover, the shift towards remote monitoring and interaction motivates CRO teams to oversee trials through digital means. In pursuit of this goal, technology startups are creating secure digital solutions, like blockchain ledgers, to optimize clinical trials. ConsilX, a startup based in Singapore, provides LifeLedger, a platform for managing patient data and events for clinical trials that utilizes blockchain technology. LifeLedger ensures regulatory compliance and data security through event-tracking and time-stamping patient activities, along with encrypting data storage. The startup's intelligent contracts facilitate the handling of cryptographic patient identities and implement rules management for adherence to regulations. ConsilX's platform, built on blockchain technology, links all participants and systems involved in a clinical trial, forming a cohesive trial management system that reduces errors and enhances efficiency.
5. Halo Health – Patient Engagement Model
Patient involvement is among the most crucial elements of clinical trials. At present, pharmaceutical and biotechnology firms invest a significant sum to oversee patient care. The difficulties here include patient recruitment and retention, as an increased number of dropouts could lead to the failure of the clinical study. This compels CRO and clinical R&D teams to adopt patient-focused strategies to lessen the strain on patients and interact with them more efficiently – right through to the trial's successful conclusion. Halo Health is a US startup focused on facilitating patient-centered smart clinical trials to alleviate burdens on patients and trial sites. The startup's model for patient engagement enables clinical trial participants to reduce their visits to trial locations and stay on track with their medication regimens. Halo’s solution simplifies the complexities of clinical trials by enabling patients to obtain 24/7 assistance and experience symptom-free days (SFD).
Top 17 Companies Leading the Clinical Trials Market
- Charles River Laboratory
- ICON Plc
- Wuxi AppTec Inc
- SGS SA
- Chiltern International Ltd
- Eli Lilly and Company
- Omnicare
- Kendle
- LabCorp
- IQVIA
- Novo Nordisk A/S
- Pfizer
- Clinipace
- Syneos Health
- PAREXEL International Corporation
- Pharmaceutical Product Development, LLC
- The Emmes Company, LLC
1. Charles River Laboratory
Headquartered in Wilmington, Massachusetts, USA
Charles River Laboratory is a global provider of preclinical and clinical laboratory services for the pharmaceutical, medical device, and biotechnology industries. The company supports the drug discovery and development process through a comprehensive portfolio that includes safety assessment, efficacy testing, and laboratory animal models. Charles River is widely recognized for its scientific expertise, regulatory knowledge, and operational excellence. Its integrated, end-to-end solutions help clients accelerate drug development timelines, reduce risk, and enhance product safety and effectiveness, playing a critical role in advancing global health outcomes.
2. ICON Plc
Headquartered in Dublin, Ireland
ICON Plc is a leading global provider of outsourced development and commercialisation services to the pharmaceutical, biotechnology, and medical device industries. ICON offers a full range of services from clinical development to post-approval support. The company is known for its innovative approach, applying data-driven insights, advanced analytics, and digital tools to enhance trial performance and reduce development timelines. With a strong presence in over 40 countries, ICON collaborates with top-tier pharma clients to bring life-changing treatments to market, while ensuring compliance with regulatory standards and maintaining high ethical research practices.
3. WuXi AppTec Inc
Headquartered in Shanghai, China
WuXi AppTec Inc, is a global pharmaceutical, biotechnology, and medical device company that provides comprehensive R&D and manufacturing services. The company offers a broad portfolio covering small molecule, biologics, and cell and gene therapy development. WuXi AppTec enables its clients to accelerate discovery, improve development processes, and bring innovative products to market faster and cost-effectively. Its integrated solutions, strong global infrastructure, and commitment to quality and compliance make it a preferred partner for firms worldwide seeking flexible and scalable support in preclinical testing, clinical research, and commercial manufacturing.
4. SGS SA
Headquartered in Geneva, Switzerland
SGS SA, is a leading global inspection, verification, testing, and certification company. In the life sciences sector, SGS provides a wide range of clinical research services, including early-phase studies, bioanalysis, and regulatory support. The company has a strong reputation for ensuring compliance, accuracy, and safety in every phase of drug development. With a global network of laboratories and offices, SGS supports pharmaceutical and biotech companies in conducting efficient and compliant clinical trials. Its reliable data delivery and regulatory expertise make it a trusted name in clinical and pharmaceutical research services.
5. Chiltern International Ltd
Headquartered in Slough, United Kingdom
Chiltern International Ltd was a prominent global Contract Research Organization (CRO), before being acquired by LabCorp’s Covance in 2017. Chiltern specialized in providing clinical development, medical and regulatory affairs, and data management services for the pharmaceutical, biotech, and medical device industries. Known for its client-centric approach and therapeutic expertise, Chiltern offered customized solutions across all phases of clinical trials. Its acquisition enhanced LabCorp’s global service capabilities, combining operational strengths to support faster, more efficient product development. Though now integrated into Covance, Chiltern’s legacy remains influential in the clinical trials sector.
Are you ready to discover more about the clinical trials market?
The report provides an in-depth analysis of the leading companies operating in the global clinical trials market. It includes a comparative assessment based on their product portfolios, business overviews, geographical footprint, strategic initiatives, market segment share, and SWOT analysis. Each company is profiled using a standardized format that includes:
Company Profiles
- Charles River Laboratory.
- Business Overview
- Company Snapshot
- Products Overview
- Company Market Share Analysis
- Company Coverage Portfolio
- Financial Analysis
- Recent Developments
- Merger and Acquisitions
- SWOT Analysis
- ICON Plc
- Wuxi AppTec Inc
- SGS SA
- Chiltern International Ltd
- Eli Lilly and Company
- Omnicare
- Kendle
- LabCorp
- IQVIA
- Novo Nordisk A/S
- Pfizer
- Clinipace
- Syneos Health
- PAREXEL International Corporation
- Pharmaceutical Product Development, LLC
- The Emmes Company, LLC
Conclusion
The clinical trials market is undergoing a transformative phase, driven by advancements in digital health, increasing focus on personalized medicine, and evolving regulatory landscapes. Innovations like decentralized trials, AI-powered recruitment, and real-world data integration are reshaping how trials are conducted, making them more efficient and patient-centric. Regions such as North America and Asia Pacific are emerging as key contributors due to their strong infrastructure and growing patient populations. As stakeholders continue to prioritize research and development, the market is poised for sustained growth, offering immense opportunities for pharmaceutical companies, CROs, and healthcare innovators across the globe.
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Need help to buy this report?