
Top 35 Companies in Biologics Safety Testing Market 2025: Strategic Overview and Future Trends (2024–2035)
RELEASE DATE: Sep 2025 Author: Spherical InsightsRequest Free Sample Speak to Analyst
Description
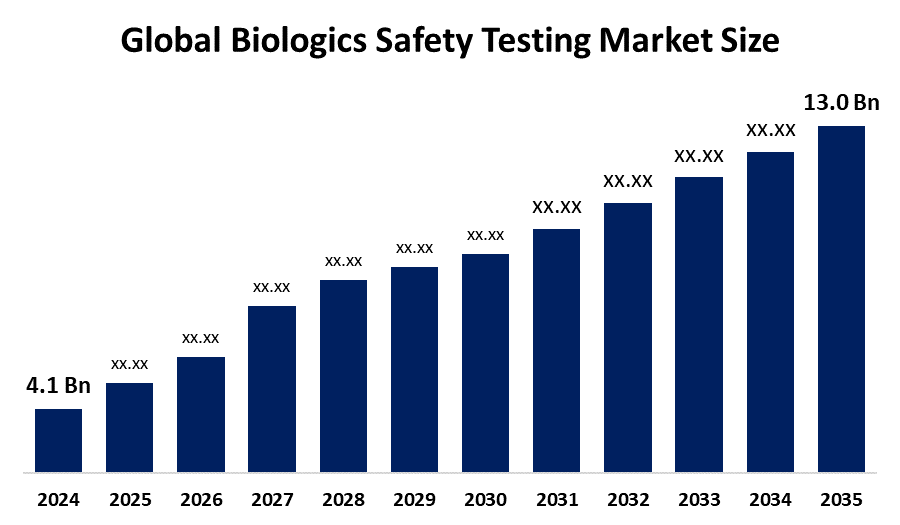
According to a research report published by Spherical Insights & Consulting, The Global Biologics Safety Testing Market Size is projected To Grow from USD 4.1 Billion in 2024 to USD 13.0 Billion by 2035, at a CAGR of 11.06% during the forecast period 2025–2035. The biologics safety testing market is driven by the rising prevalence of chronic diseases, rising biopharmaceutical development, and increasing approvals of biologics and biosimilars.
Introduction
The Biologics Safety Testing Market Size is the global industry that analyzes and characterizes biological products to verify their safety, effectiveness, and regulatory compliance. This includes vaccines, gene treatments, monoclonal antibodies, biosimilars, and other biopharmaceuticals that must undergo rigorous testing to detect and remove impurities, confirm identity and potency, and validate overall safety. Biologics safety testing is vital in establishing the absence of bacterial or viral contamination, therefore safeguarding patients and maintaining the trustworthiness of treatments. The market is quickly rising due to the growing number of biopharmaceutical businesses generating innovative therapeutic medications, which has increased the emphasis on improving industrial processes and guaranteeing quality control. Globally, stringent regulatory regulations encourage the usage of sophisticated testing tools and services. Furthermore, advancements in next-generation therapies, which aim to improve efficacy, accuracy, and safety, are generating new revenue potential. As biologics have become the dominant class of therapeutics, biologics safety testing has evolved as a critical facilitator of product development and commercialization, assuring safe, effective, and compliant treatments for a wide variety of medical uses.
Navigate Future Markets with Confidence: Insights from Spherical Insights LLP
The insights presented in this blog are derived from comprehensive market research conducted by Spherical Insights LLP, a trusted advisory partner to leading global enterprises. Backed by in-depth data analysis, expert forecasting, and industry-specific intelligence, our reports empower decision-makers to identify strategic growth opportunities in fast-evolving sectors. Clients seeking detailed market segmentation, competitive landscapes, regional outlooks, and future investment trends will find immense value in the full report. By leveraging our research, businesses can make informed decisions, gain a competitive edge, and stay ahead in the transition toward sustainable and profitable solutions.
Unlock exclusive market insights - Download the Brochure now and dive deeper into the future of the Biologics Safety Testing Market.
Biologics Safety Testing Market Size & Statistics
- The Market Size for Biologics Safety Testing Was Estimated to be worth USD 4.1 Billion in 2024.
- The Market Size is Going to Expand at a CAGR of 11.06% between 2025 and 2035.
- The Global Biologics Safety Testing Market Size is anticipated to reach USD 13.0 Billion by 2035.
- North America is expected to generate the highest demand during the forecast period in the Biologics Safety Testing Market
- Asia Pacific is expected to grow the fastest during the forecast period in the Biologics Safety Testing Market.
Regional growth and demand
Asia Pacific is expected to grow the fastest during the forecast period in the Biologics Safety Testing market. Asia Pacific is expected to register the fastest CAGR in the biologics safety testing market, fueled by rising biopharmaceutical investments, strong development of monoclonal antibodies and biosimilars, and increasing R&D activities aimed at advancing the region’s biotechnology sector and biologics innovation.
North America is expected to generate the highest demand during the forecast period in the Biologics Safety Testing market. North America is projected to hold the largest share of the biologics safety testing market, driven by stringent USFDA regulations, rising demand for biologics such as mAbs, vaccines, and cell therapies, and the increasing prevalence of chronic diseases requiring rigorous safety assessments.
Top 10 Trends in the Biologics Safety Testing Market
- Expansion of Biosimilars
- Technological Advancements
- Increased Outsourcing
- Strict Regulatory Requirements
- Growth in ATMPs
- Rising Demand for Biologics
- Addressing Cell Culture Contamination
- Increased R&D Investment
- Focus on Emerging Markets
- Shift to Non-Animal Testing
1. Expansion of Biosimilars
The increasing demand for low-cost biologics has resulted in significant expansion in the biosimilar market. As biosimilars reach global markets, stringent safety testing is essential to guarantee they fulfill quality, safety, and effectiveness criteria, resulting in increased demand for sophisticated biologics safety testing solutions.
2. Technological Advancements
Safety testing is being transformed by innovations like as automation, real-time monitoring, high-throughput screening, and AI-driven data analysis. These innovations increase speed, accuracy, and efficiency while decreasing human error, allowing biopharma businesses to expedite biologics development while maintaining safety and compliance.
3. Increased Outsourcing
Biopharmaceutical firms are increasingly delegating safety testing to contract research organizations (CROs) and specialized labs. This trend is driven by cost reductions, access to modern testing methods, and the knowledge needed to fulfill global regulatory requirements.
4. Strict Regulatory Requirements
Global agencies such as the US FDA and the European Medicines Agency impose strict biologics safety requirements. These laws drive up demand for comprehensive testing solutions that ensure biologics are free of contaminants and safe for patients, ultimately influencing industry practices.
5. Growth in ATMPs
The development of cell treatments, gene therapies, and other sophisticated therapeutics has posed new hurdles for safety evaluation. ATMPs require sophisticated biologics safety testing methodologies to certify their purity, potency, and safety, resulting in tremendous market potential.
Empower your strategic planning:
Stay informed with the latest industry insights and market trends to identify new opportunities and drive growth in the biologics safety testing market. To explore more in-depth trends, insights, and forecasts, please refer to our detailed report.
Top 25 Companies Leading the Biologics Safety Testing Market
- Abcam
- PathogenDx
- Thermo Fisher Scientific
- Boehringer Ingelheim
- Vancouver Testing Laboratories
- Sartorius AG
- Wuxi AppTec
- Lonza Group
- Merck KGaA
- SGS SA
- Charles River Laboratories
- Invetech
- BioReliance
- Eurofins Scientific
- WuXi PharmaTech
- FUJIFILM
- F.Hoffmann-La Roche Ltd
- BIOMERIEUX
- Maravai LifeSciences.
- Sotera Health
- Samsung Biologics
- GenScript
- Agilent Technologies Inc.
- Syngene International Limited
- Laboratory Corporation of America
1. Abcam
Headquarters: Cambridge, United Kingdom
Abcam has 13 locations and serves clients in more than 130 countries, with a strong focus on delivering antibodies, proteins, reagents, and assays for important research and testing. The company's products are widely used in biologics safety testing to assist precise contamination identification, product purity verification, and assay reliability evaluation. By providing critical testing components, Abcam assists pharmaceutical and biotechnology firms in meeting strict safety standards and regulatory requirements for biologics research and manufacturing.
2. PathogenDx
Headquarters: Scottsdale, Arizona, USA
PathogenDx, located in Scottsdale, Arizona, operates largely in North America but is growing globally. The firm specializes in DNA-based pathogen detection solutions, including its unique D³ Array technology, which allows for quick, multiplexed testing. In the context of biological safety testing, these technologies are used to detect microbial contamination with great precision and speed, assuring the safety and quality of biopharmaceuticals. PathogenDx's focus on molecular diagnostics innovation improves the safety evaluations necessary for biologics, vaccines, and biosimilars in regulated healthcare markets.
3. THERMO FISHER SCIENTIFIC
Headquarters: Waltham, Massachusetts, USA
Thermo Fisher Scientific, based in Waltham, Massachusetts, operates in over 60 countries and has one of the most comprehensive portfolios of laboratory instruments, consumables, and testing services. Within biologics safety testing, the business offers proven solutions for endotoxin testing, sterility testing, residual DNA analysis, and contamination identification. These techniques are essential for ensuring the safety, effectiveness, and regulatory compliance of biopharmaceuticals, vaccines, and advanced medicines. Thermo Fisher's worldwide presence and comprehensive product range position it as a key leader in biologics development and large-scale quality assurance.
4. Boehringer Ingelheim
Headquarters: Ingelheim, Germany
Boehringer Ingelheim, based in Ingelheim, Germany, has 146 subsidiaries in 78 countries and focuses primarily on human medications, animal health, and biopharmaceutical contract manufacturing. Its biologics portfolio comprises monoclonal antibodies, vaccines, and advanced medicines, all of which need rigorous biologics safety testing to meet regulatory requirements. The organization uses modern testing methods to verify product purity, sterility, and safety throughout the development process. Boehringer Ingelheim fosters innovation in varied healthcare markets by incorporating biologics safety testing into its global operations.
5. Vancouver Testing Laboratories
Headquarters: Vancouver, Canada
Vancouver Testing Laboratories, based in Vancouver, Canada, offers specialized microbiological and environmental safety testing services. The firm primarily serves clients in North America but also opts for international collaborations. It provides bioburden analysis, endotoxin testing, and sterility evaluations, all of which are critical to biologics safety. Its services help pharmaceutical and biotechnology industries evaluate product purity and achieve stringent safety standards during biologics manufacture. By concentrating on quality control and regulatory compliance, Vancouver Testing Laboratories assists the biopharmaceutical sector in providing safe, dependable vaccines, biosimilars, and innovative therapies to worldwide markets.
Are you ready to discover more about the biologics safety testing market?
The report provides an in-depth analysis of the leading companies operating in the global biologics safety testing market. It includes a comparative assessment based on their product portfolios, business overviews, geographical footprint, strategic initiatives, market segment share, and SWOT analysis. Each company is profiled using a standardized format that includes:
Company Profiles
- Abcam
- Business Overview
- Company Snapshot
- Products Overview
- Company Market Share Analysis
- Company Coverage Portfolio
- Financial Analysis
- Recent Developments
- Merger and Acquisitions
- SWOT Analysis
- PathogenDx
- THERMO FISHER SCIENTIFIC
- Boehringer Ingelheim
- Vancouver Testing Laboratories
- Sartorius AG
- Wuxi AppTec
- Lonza Group
- Merck KGaA
- Others.
Conclusion
The biologics Safety Testing Market Size is expanding steadily, driven by increased demand for biologics, biosimilars, vaccines, and advanced treatments. Stringent regulatory requirements, combined with the necessity for reliable contaminant identification and assurance of product purity, are driving the use of sophisticated safety testing methodologies. Market growth is being driven by increased R&D spending, technical improvements including automation, AI, and high-throughput screening, as well as the outsourcing of testing services. With the increasing proliferation of biosimilars and ATMPs, biologics safety testing is still required to ensure effectiveness, safety, and compliance, ensuring its crucial position in global healthcare development.
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Need help to buy this report?