Global Vutrisiran Market Size, Share, and COVID-19 Impact Analysis, By Drug Class (Antisense Oligonucleotide, Nucleic Acids, Nucleotide, Nucleosides, & Others), By Route of Administration (Injectable, Parenteral, Subcutaneous), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, & Others), By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2022 - 2032
Industry: HealthcareGlobal Vutrisiran Market Insights Forecasts to 2032
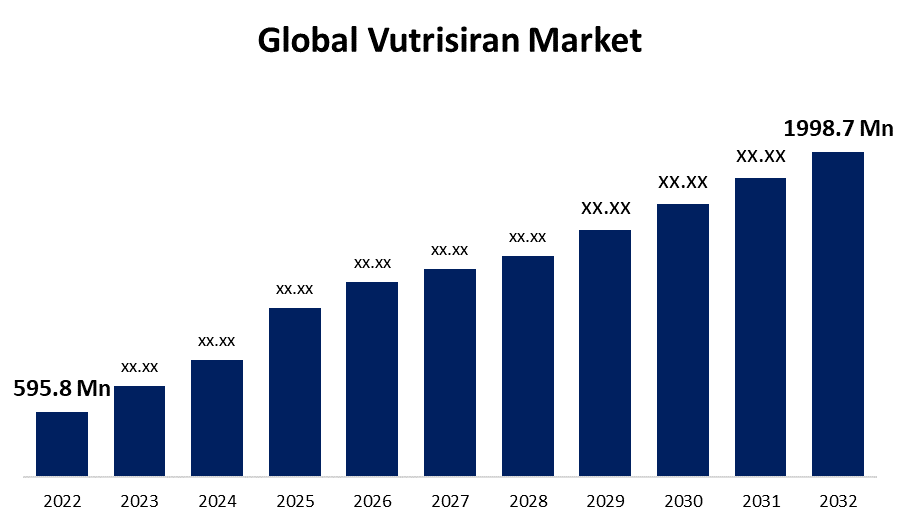
- The Global Vutrisiran Market Size was valued at USD 595.8 Million in 2022.
- The Market is growing at a CAGR of 12.8% from 2022 to 2032.
- The Worldwide Vutrisiran Market size is expected to reach USD 1,998.7 Million by 2032.
- Europe is expected to grow the fastest during the forecast period.
Get more details on this report -
The Global Vutrisiran Market Size is expected to reach USD 1,998.7 Million by 2032, at a CAGR of 12.8% during the forecast period 2022 to 2032. Increased healthcare spending, research and development activities, increased awareness, and advances in healthcare services all have a favorable impact on the Vutrisiran market. Additionally, the development of novel medicinal items provides market participants with attractive chances during the projection period.
Market Overview
Vutrisiran is a medication for hereditary transthyretin-mediated amyloidosis (hATTR), a rare and progressive genetic disease that affects various organs and tissues, including the nerves, heart, and kidneys. The medication works by silencing the production of the protein responsible for the aberrant accumulation of amyloid fibrils in the body utilizing RNA interference (RNAi) technology. Vutrisiran was approved by the US Food and Drug Administration (FDA) in September 2021 along with the European Medicines Agency (EMA) in November 2021. It's currently available in the United States, Europe, and other regions around the globe. Vutrisiran, a chemically modified double-stranded siRNA discovered in AMVUTTRA, uses RNA interference to target mutant and wild-type TTR messenger RNA (mRNA), resulting in their destruction and a decrease in serum TTR protein levels. A distinguishing feature of hATTR is the extracellular deposition of misfolded transthyretin (TTR) protein. The condition can impact a variety of organs and systems, including the liver, neurological system, heart, kidneys, and gastrointestinal tract. Vutrisiran is covalently linked to a ligand with three N-acetyl galactosamine (GalNAc) residues to transport siRNA to hepatocytes.
Report Coverage
This research report categorizes the global vutrisiran market based on various segments and regions and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global vutrisiran market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global vutrisiran market. Technological innovation and advancement will further optimize the performance of the product, enabling it to acquire a wider range of applications in the downstream market.
Global Vutrisiran Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2022 |
| Market Size in 2022: | USD 595.8 Million |
| Forecast Period: | 2022 – 2032 |
| Forecast Period CAGR 2022 – 2032 : | 12.8% |
| 022 – 2032 Value Projection: | USD 1998.7 Million |
| Historical Data for: | 2018-2021 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Drug Class, By Route of Administration, By Distribution Channel, By Region, COVID-19 Impact Analysis |
| Companies covered:: | Alnylam Pharmaceuticals, Inc., Orphalan, AstraZeneca, Vivet Therapeutics, Pfizer Inc., Ultragenyx Pharmaceutical, Valeant Pharmaceuticals International, Inc., Noble Pharma Co., Ltd., Merck & Co., Inc., Teva Pharmaceuticals |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The rising prevalence of chronic diseases is the key driver of this therapeutic industry. Furthermore, an increase in the number of people suffering from transthyretin amyloidosis around the world is one of the important factors driving revenue growth in the vutrisiran market. As a result of improved diagnosis and increased disease awareness, the prevalence of hATTR is developing. This pattern is expected to continue in the coming years, most likely resulting in a larger patient pool for vutrisiran. Vutrisiran's recent FDA and EMA regulatory approvals have expanded the medication's availability to more locations, which may drive demand for the treatment. Hereditary transthyretin-mediated amyloidosis (hATTR) is a rare and progressive hereditary disease having limited therapy options at the moment. Vutrisiran, a new therapy approach that targets the disease's fundamental cause, demonstrated promising outcomes in clinical trials. As a result, there is a significant unmet medical need for effective hATTR therapies, which may boost the demand for Vutrisiran. Also, the increase in clinical trials for improved drugs and therapies, as well as the focused focus on transthyretin amyloidosis research and development, are driving market revenue growth.
Restraining Factors
The Vutrisiran market's revenue growth is likely to be further hampered in the coming years due to a lack of qualified employees. However, it is anticipated that a lack of diagnosis and the high cost of therapy will limit the revenue from the market growth. Vutrisiran is an expensive drug that requires close monitoring of patients to ensure its safety and effectiveness which may restrict the market growth of the vutrisiran.
Market Segmentation
- In 2022, the antisense oligonucleotide segment is dominating the market with the largest market share during the forecast period.
The global vutrisiran market is segmented into antisense oligonucleotide, nucleic acids, nucleotide, nucleosides, & others, based on the type. Among these, the antisense oligonucleotide segment is projecting significant growth owing to the antisense oligonucleotides (ASOs) having emerged as a promising class of medications for treating a variety of genetic and uncommon disorders, including hATTR. Antisense oligonucleotides are synthetic compounds that attach to specific messenger RNAs (mRNAs) and alter their expression or function, thereby lowering the production of disease-causing proteins. The US FDA has already approved several antisense oligonucleotides for the treatment of conditions such as spinal muscular atrophy and Duchenne muscular dystrophy.
- In 2022, the subcutaneous segment is influencing the largest market share over the forecast period.
Based on the route of administration, the global vutrisiran market is divided into oral and injectable, & subcutaneous. During the projected period, the subcutaneous segment will hold the greatest market share owing to the vutrisiran will most likely be administered via subcutaneous injection, depending on the drug's mechanism of action and formulation. This is because vutrisiran is an RNA-based therapy, specifically an RNA interference (RNAi) molecule designed to limit the synthesis of aberrant transthyretin (TTR) protein in individuals with hATTR. To be successful, RNAi molecules must often be delivered to specific tissues or cells, and subcutaneous injection is a frequent mode of delivery for many RNAi-based medications. Furthermore, subcutaneous injection is a practical and relatively straightforward method of drug administration, and many patients can be taught to self-administer subcutaneous injections at home.
- In 2022, the hospital pharmacies segment is driving market share growth during the forecast period.
On the basis of distribution channels, the global vutrisiran market is segmented into hospital pharmacies, retail pharmacies, online pharmacies, & others. Among these, hospital pharmacies are expected to grow the market during the forecast period, owing to hospital pharmacies specializing in managing and dispensing drugs used in the hospital setting, including high-cost medications like Vutrisiran. The US FDA has already approved several antisense oligonucleotides for the treatment of conditions such as spinal muscular atrophy and Duchenne muscular dystrophy. These materials are available to hospital pharmacies, which may ensure that Vutrisiran is appropriately administered to patients. Because hospital pharmacies have access to patient medical data and can collaborate closely with healthcare practitioners to monitor patients' responses to Vutrisiran, they can provide this level of monitoring and support. Also, the hospital pharmacies can collaborate closely with other healthcare providers, such as physicians, nurses, and other support workers, to ensure that patients receiving Vutrisiran receive the best possible treatment. Thus, hospital pharmacies are projected to hold 46% of the worldwide vutrisiran market in 2023.
Regional Segment Analysis of the vutrisiran market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
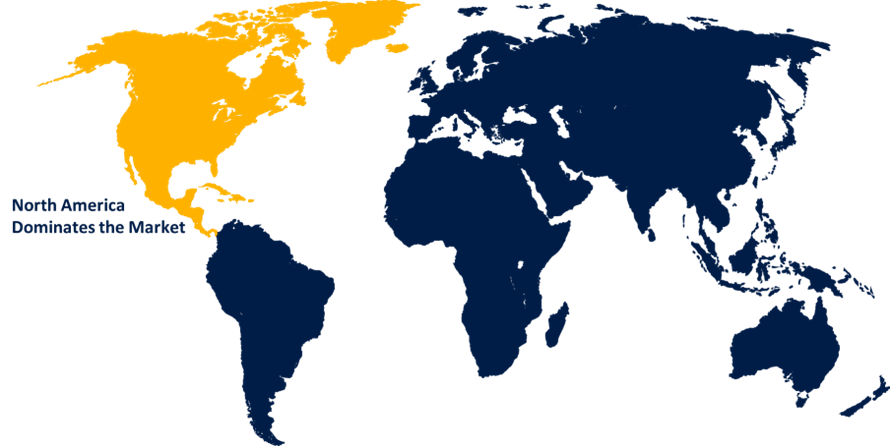
North America is holding the market with the largest market share over the forecast period
Get more details on this report -
North America is predicted to register the highest revenue growth during the forecast period, because of the presence of key manufacturers and the increased number of diagnostic procedures conducted in the region, the market in North America is predicted to register the highest revenue growth during forecast period. Moreover, the factors such as the high prevalence of hATTR, the availability of favorable reimbursement policies, & Alnylam Pharmaceuticals, the company that developed Vutrisiran, A robust pipeline of RNAi-based medicines might help the growth of the Vutrisiran market in North America.
Europe is expected to register significant revenue growth during the forecast period, owing to the firm that created Vutrisiran, Alnylam Pharmaceuticals, which has a robust pipeline of other RNAi-based medicines under development for a variety of disorders. The success of Vutrisiran could boost investor confidence in the company and enhance funding for its other pipeline medications, resulting in the rise of the vutrisiran market in Europe.
Competitive Analysis
The report offers the appropriate analysis of the key organizations/companies involved within the global vutrisiran market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Alnylam Pharmaceuticals, Inc.
- Orphalan
- AstraZeneca
- Vivet Therapeutics
- Pfizer Inc.
- Ultragenyx Pharmaceutical
- Valeant Pharmaceuticals International, Inc.
- Noble Pharma Co., Ltd.
- Merck & Co., Inc.
- Teva Pharmaceuticals
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In June 2022, Alnylam Pharmaceuticals has set up patient access programs to guarantee that vutrisiran is available to patients who require it. These programs provide patients with financial assistance, support services, and other resources to help them access and afford vutrisiran.
- In July 2021, The European Medicines Agency (EMA) approved Vutrisiran for the treatment of hereditary transthyretin-mediated amyloidosis (hATTR) in adults with polyneuropathy. This has given Vutrisiran access to the European market, which is projected to drive market growth.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2022 to 2032. Spherical Insights has segmented the Global Vutrisiran Market based on the below-mentioned segments:
Global Vutrisiran Market, By Drug Class
- Antisense Oligonucleotide
- Nucleic Acids
- Nucleotide
- Nucleoside
- Others
Global Vutrisiran Market, By Route of Administration
- Oral
- Injectable
- Subcutaneous
Global Vutrisiran Market, By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Global Vutrisiran Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of Middle East & Africa
Need help to buy this report?