United States Pharmacovigilance Market Size, Share, and COVID-19 Impact Analysis, By Source (Literature Reports, Scheduled Reporting, and Spontaneous Reporting), By Deployment Mode (On-Premise and Cloud-Based), By Service Type (Data Mining, Adverse Event Reporting, Pharmacovigilance Consulting, and Risk Assessment), By End-User (Biotechnology Companies and Pharmaceutical Companies), and US Pharmacovigilance Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareUSA Pharmacovigilance Market Insights Forecasts to 2035
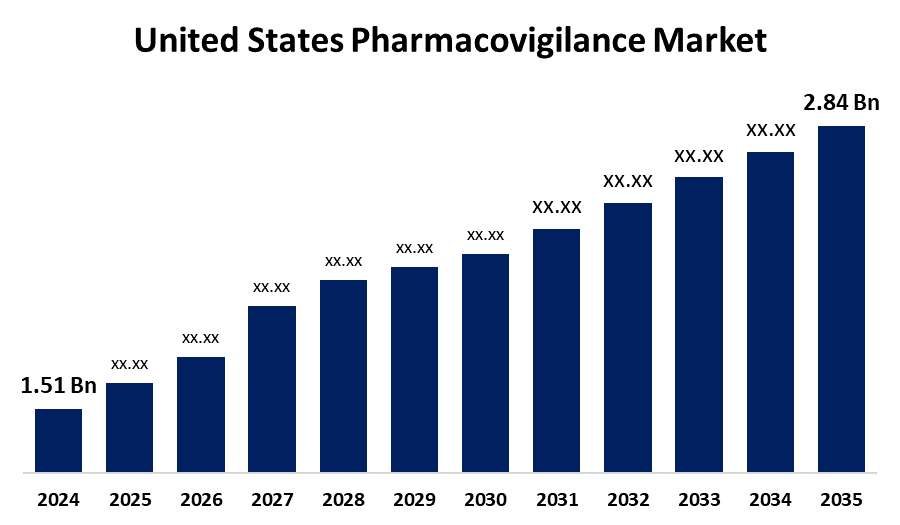
- The US Pharmacovigilance Market Size was Estimated at USD 1.51 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 5.91% from 2025 to 2035
- The USA Pharmacovigilance Market Size is Expected to reach USD 2.84 Billion by 2035
Get more details on this report -
According to a research report published by Spherical Insights and Consulting, The US Pharmacovigilance Market Size is anticipated to reach USD 2.84 Billion by 2035, growing at a CAGR of 5.91% from 2025 to 2035. The market growth is owing to the rising prevalence of acute and chronic diseases, growing approval from regulatory authorities for medications, and increasing cases of adverse effects due to consumption of the medications for the long-term or repeated use.
Market Overview
The US pharmacovigilance market ensures drug safety and regulatory compliance under FDA supervision by monitoring, identifying, and preventing adverse drug reactions and medication-related risks. Pharmacovigilance is the science of identifying, evaluating, comprehending, and averting side effects or issues associated with medications or vaccines. It has expanded rapidly as a result of greater awareness among medical professionals. Resolving medicolegal cases in which a person requires medical attention for injuries or harm requires its components, such as identifying and preventing adverse drug reactions (ADRs). These cases, which include homicides, suicides, and accidents, are appearing in the modern medical field at a startling rate. Pharmacovigilance is regulated by the FDA and the U.S. Department of Health and Human Services, with assistance from the Center for Biologics Evaluation and Research (CBER) and the Center for Drug Evaluation and Research (CDER). Title 21 of the FDA Code of Federal Regulations (CFR) and the Federal Food, Drug, and Cosmetic Act (FDCA) govern the regulatory framework. CDER supervises pharmacovigilance initiatives to guarantee that medications on the market are safe and effective.
Report Coverage
This research report categorizes the market for the US pharmacovigilance market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the US pharmacovigilance market. Recent market developments and competitive strategies such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the US pharmacovigilance market.
United States Pharmacovigilance Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 1.51 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR Of 5.91% |
| 2035 Value Projection: | USD 2.84 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 288 |
| Tables, Charts & Figures: | 104 |
| Segments covered: | By Source, By Deployment Mode, By Service Type and By End-User |
| Companies covered:: | IQVIA, Celerion, Aris Global, Syneos Health, Medpace, Oracle, Parexel, Bioclinica, Wuxi App Tec, Covance, and Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Growing trend of artificial intelligence in the pharmacovigilance sector:
Drug safety monitoring procedures are being revolutionized by artificial intelligence (AI), which can recognize trends and possible adverse events in large amounts of data. Additionally, this technology increases PV professionals' productivity, enabling them to do more value-based work. AI enhances adverse event detection and evaluation, enabling regulatory bodies, PV specialists, and pharmaceutical corporations to make more educated decisions. The introduction of AI-powered solutions by major companies to transform these service offerings is expected to enhance market growth. For instance, the US FDA has launched the Emerging Drug Safety Technology Program (EDSTP), a program focusing on the use of AI and other emerging technologies in pharmacovigilance. This initiative is part of the Center for Drug Evaluation and Research's (CDER) comprehensive approach to enhance mutual learning about AI usage throughout the drug product lifecycle.
Multi-drug therapy, intricacy in clinical research, and rising demand for pharmacovigilance services accelerate the market expansion:
The growing complexity of clinical trials and the prevalence of chronic diseases, which necessitate the use of many therapeutic drugs concurrently by patients, are driving expansion in the pharmacovigilance industry. ADR monitoring is essential since this raises the safety risk. Pharmacovigilance responsibilities are increasingly being outsourced by pharmaceutical corporations to professional service providers that give access to regional regulatory intelligence, scalable infrastructure, and skilled analysts. The increasing difficulty of creating new drugs, especially in the fields of biologics, gene treatments, and personalized medicine, is the factor that drives the market growth.
Restraining Factors
The regulatory complexity, high implementation costs, data management issues, a shortage of experienced workers, fierce rivalry, and high implementation costs regulatory restrictions make operations expensive and time-consuming, and data management difficulty, which limits the market growth.
Market Segmentation
The USA pharmacovigilance market share is classified into source, deployment mode, service type, and end-user.
- The spontaneous reporting segment held a substantial share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The US pharmacovigilance market is segmented by source into literature reports, scheduled reporting, and spontaneous reporting. Among these, the spontaneous reporting segment held the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segment growth is attributed to the active reporting method, which detects and monitors the safety signals of the drugs and is cost-effective.
- The on-premise segment accounted for the largest share in 2024 and is predicted to grow at a significant CAGR during the forecast period.
The US pharmacovigilance market is segmented by deployment mode into on-premise and cloud-based. Among these, the on-premise segment accounted for the largest share in 2024 and is predicted to grow at a significant CAGR during the forecast period. The sector growth is attributed to the software can be placed on an organization's internal servers using the on-premises deployment technique, which gives complete control over the system and data. Despite greater initial and ongoing costs, it is the favored option for businesses managing sensitive data and requiring strict security measures.
- The adverse event reporting segment held the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The US pharmacovigilance market is segmented by service type into data mining, adverse event reporting, pharmacovigilance consulting, and risk assessment. Among these, the adverse event reporting segment held the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segmental expansion is ascribed to identify the potential risks, improve patient safety.
- The pharmaceutical companies segment accounted for a substantial share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The US pharmacovigilance market is segmented by end-user into biotechnology companies and pharmaceutical companies. Among these, the pharmaceutical companies segment accounted for a substantial share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segment growth is attributed to collecting the description and information on adverse effects and ensuring the risk with the regulatory compliance.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the US pharmacovigilance market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- IQVIA
- Celerion
- Aris Global
- Syneos Health
- Medpace
- Oracle
- Parexel
- Bioclinica
- Wuxi App Tec
- Covance
- Others
Recent Developments:
- In March 2025, IT services firms Wipro, TechMahindra, and L&T Technology have partnered with chipmaker Nvidia for AI-based solutions across various industries. Tech Mahindra has introduced an autonomous pharmacovigilance solution powered by Nvidia AI software, improving drug safety management speed, accuracy, and efficiency by addressing manual delays and data overload.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the United States, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the US pharmacovigilance market based on the below-mentioned segments:
US Pharmacovigilance Market, By Source
- Literature Reports
- Scheduled Reporting
- Spontaneous Reporting
US Pharmacovigilance Market, By Deployment Mode
- On-Premise
- Cloud-Based
US Pharmacovigilance Market, By Service Type
- Data Mining
- Adverse Event Reporting
- Pharmacovigilance Consulting
- Risk Assessment
US Pharmacovigilance Market, By End-User
- Biotechnology Companies
- Pharmaceutical Companies
Need help to buy this report?