United States Perjeta Market Size, Share, and COVID-19 Impact Analysis, By Product (Monoclonal Antibody and Generic Drug), By Indication (Early Breast Cancer and Metastatic Breast Cancer), and United States Perjeta Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareUnited States Perjeta Market Insights Forecasts to 2035
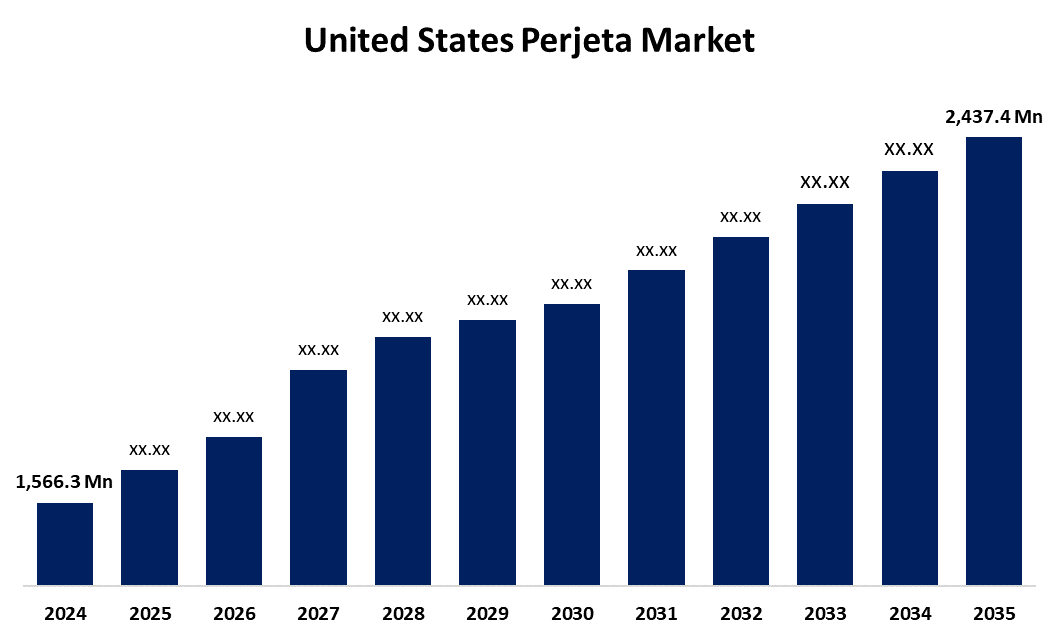
- The US Perjeta Market Size Was Estimated at USD 1,566.3 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 4.10% from 2025 to 2035
- The US Perjeta Market Size is Expected to Reach USD 2,437.4 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the United States Perjeta Market Size is anticipated to reach USD 2,437.4 Million by 2035, Growing at a CAGR of 4.10% from 2025 to 2035. The rising incidence of HER2-positive breast cancer propels the expansion of the United States perjeta market. Improved diagnostics and early detection have made it easier to identify qualified individuals, increasing the number of available treatments.
Market Overview
A recombinant humanised monoclonal antibody is called Perjeta. HER2-positive breast cancer, defined by the excess of HER2 proteins on cancer cells, is the primary indication for which it is used. One of the novel monoclonal antibody therapies for HER2-positive breast cancer is Perjeta (pertuzumab). This dual-targeted approach not only provides a better clinical outcome than trastuzumab (Herceptin) in both neoadjuvant and metastatic treatment settings, but the original biologic agents preserve their unique therapeutic value and clinical uniqueness, despite the availability of potential biosimilar products at a lower overall cost. The main driver of the industry's positive outlook is the growing incidence of breast cancer. A sizeable proportion of all breast cancer cases is HER2-positive breast cancer, which is a particularly aggressive type of breast cancer. Increased understanding, greater diagnostic capability, and new screening initiatives mean an increasing frequency of diagnosing HER2-positive individuals. The increasing volume of breast cancer will drive an increasing demand for treatments such as Perjeta, which is an important breast cancer drug in current oncology practices. Perjeta's great safety profile and demonstrated survival benefits are the reason for its demand. Perjeta (pertuzumab) plus trastuzumab and chemotherapy decreased death risk by 17% over 10 years in HER2+ early breast cancer patients compared with trastuzumab/chemotherapy alone, according to the final APHINITY study data released by Roche and research partners in May 2025. By combining pertuzumab with trastuzumab, its dual blocking method provides a more thorough inhibition of the HER2 receptor, enhancing treatment results. Perjeta has gained a significant position in neoadjuvant and metastatic treatment settings due to its apparent therapeutic benefits. Strong clinical evidence demonstrating its long-term effectiveness further supports its use and trust among oncologists and other healthcare professionals.
Report Coverage
This research report categorizes the market for the United States perjeta market based on various segments and regions, and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the United States perjeta market. Recent market developments and competitive strategies such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the United States perjeta market.
United States Perjeta Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 1,566.3 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 4.10% |
| 2035 Value Projection: | USD 2,437.4 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 199 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Product, By Indication, and COVID-19 Impact Analysis |
| Companies covered:: | Carisma Therapeutics, Ambrx Biopharma, Pfizer Inc., Genentech, Inc., F. Hoffmann-La Roche Ltd, Roche, AstraZeneca, Novartis, and Others. |
| Pitfalls & Challenges: | Covid-19 Empact, Challenges, Growth, Analysis. |
Get more details on this report -
Driving Factors
The growth of the United States perjeta market is fueled by the accessibility, practicality, and general patient experience of HER2-targeted medicines are being improved by developments in treatment delivery. Long infusion dimensions and hospitalisations are no longer necessary because of innovations like subcutaneous formulations and fixed-dose combinations, which have reduced the administration process. Roche's EU label revisions for Phesgo (pertuzumab/trastuzumab) were approved by CHMP in April 2025. This allows medical practitioners to administer the drug subcutaneously in nonclinical settings, like residences, following clinical establishment for the treatment of HER2+ breast cancer. These delivery improvements maximise the use of healthcare resources while also increasing patient happiness and compliance. As Perjeta becomes more adaptable to various clinical contexts, its usability for a wider range of patients improves, facilitating its inclusion into standard care practices and accelerating its growth trajectory.
Restraining Factors
The United States perjeta market faces obstacles because creating and manufacturing biologics is hard and requires a large investment in R&D and manufacturing capabilities. The Perjeta market has high entry barriers.
Market Segmentation
The United States perjeta market share is classified into product and indication.
- The monoclonal antibody segment held the largest market share in 2024 and is expected to grow at a remarkable CAGR during the forecast period.
The United States perjeta market is segmented by product into monoclonal antibody and generic drug. Among these, the monoclonal antibody segment held the largest market share in 2024 and is expected to grow at a remarkable CAGR during the forecast period. The segment is driven by its proven capacity to target breast cancer cells that are HER2-positive. For example, Perjeta's clinical use has been supported by the FDA's approval of its combination with chemotherapy for neoadjuvant treatment in patients with early-stage HER2-positive breast cancer. Its market position is further boosted by its capacity to amplify the effects of other HER2-targeted treatments, like trastuzumab. Oncologists and patients prefer it over standard chemotherapy because of its high specificity and fewer adverse effects. Confidence in this therapeutic strategy has increased due to recent Phase III trial data showing enhanced progression-free survival. Sales growth in this area is driven by well-established production procedures and widespread physician awareness.
- The metastatic breast cancer segment held the highest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period.
Based on the indication, the United States perjeta market is segmented into early breast cancer and metastatic breast cancer. Among these, the metastatic breast cancer segment held the highest revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segment growth is fueled by Perjeta is known to be beneficial for slowing the advancement of advanced illness. Updated data from the CLEOPATRA trial were recently published, confirming that it improves overall survival for metastatic patients incorporating docetaxel and Perjeta with trastuzumab. The process of Perjeta improves the outcome of metastatic patients by targeting resistant cancer cells. Its effectiveness and safety in later-stage cancer situations are supported by more extensive clinical trial evidence. Because doctors are looking for more focused treatment alternatives, Perjeta is a popular option for combination regimens. Market expansion and high demand are sustained by the unmet medical need in metastatic breast cancer.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the United States perjeta market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Carisma Therapeutics
- Ambrx Biopharma
- Pfizer Inc.
- Genentech, Inc.
- F. Hoffmann-La Roche Ltd
- Roche
- AstraZeneca
- Novartis
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the United States, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the United States perjeta market based on the following segments:
United States Perjeta Market, By Product
- Monoclonal Antibody
- Generic Drug
United States Perjeta Market, By Indication
- Early Breast Cancer
- Metastatic Breast Cancer
Need help to buy this report?