United States Microbiome Therapeutics Market Size, Share, and COVID-19 Impact Analysis, By Type (FMT and Microbiome Drugs), By Application (C. difficile, Crohn’s disease, Inflammatory Bowel Disease, Diabetes, and Others), and United States Microbiome Therapeutics Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareUnited States Microbiome Therapeutics Market Insights Forecasts to 2035
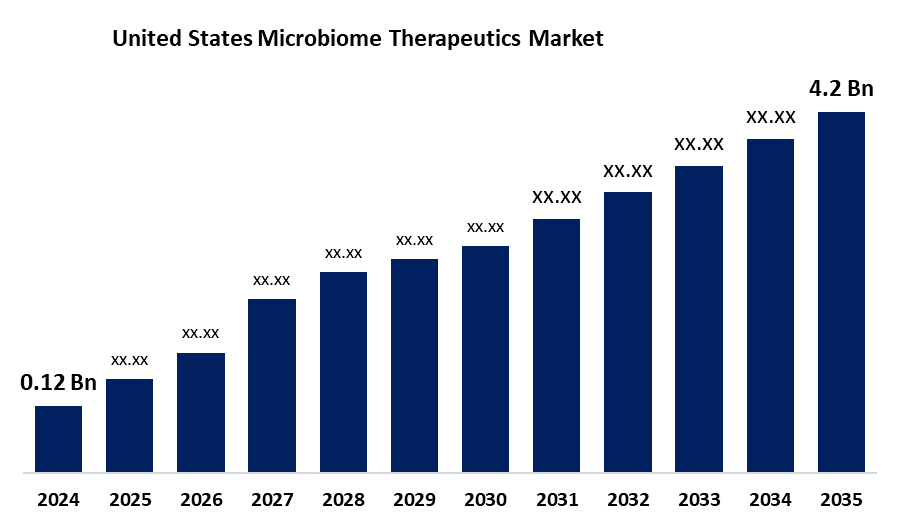
- The Japan Microbiome Therapeutics Market Size was estimated at USD 0.12 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 38.16% from 2025 to 2035
- The Microbiome Therapeutics Market Size is Expected to Reach USD 4.2 Billion by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the microbiome therapeutics market is anticipated to reach USD 4.2 billion by 2035, growing at a CAGR of 38.16% from 2025 to 2035. The United States microbiome therapeutics market is expanding due to increasing FDA approvals, rising prevalence of gastrointestinal disorders, and ongoing advancements in microbiome research. Strategic investments and collaborations are also accelerating the development and commercialization of innovative microbiome-based therapies, driving strong growth and greater adoption in the healthcare sector.
Market Overview
The United States microbiome therapeutics market refers to the developing treatments that utilize the human microbiome, a complex community of microorganisms within the body, to prevent and treat various diseases. These therapies include live biotherapeutic products, prebiotics, and postbiotics targeting conditions such as Clostridioides difficile infection, inflammatory bowel disease, and metabolic disorders. Market growth is driven by increasing understanding of the microbiome’s role in health, advances in sequencing technologies, and rising prevalence of chronic diseases. The market’s strengths lie in the U.S.’s robust healthcare infrastructure, significant research funding, and a favourable regulatory environment that supports innovation and commercialization. Various opportunities exist in personalized medicine, where treatments can be tailored to individual microbiomes, enhancing therapeutic efficacy. Additionally, collaborations between biotech firms and pharmaceutical companies accelerate development and expand applications. Government initiatives from agencies like the FDA and National Institutes of Health further support this growth through funding, research programs, and efforts to establish clear regulatory frameworks, fostering a conducive environment for microbiome therapeutic advancements and adoption.
Report Coverage
This research report categorizes the market for the United States microbiome therapeutics market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the USA microbiome therapeutics market. Recent market developments and competitive strategies such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the U.S. microbiome therapeutics market.
United States Microbiome Therapeutics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 0.12 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 38.16% |
| 2035 Value Projection: | USD 4.2 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 210 |
| Tables, Charts & Figures: | 126 |
| Segments covered: | By Type, By Application and COVID-19 Impact Analysis |
| Companies covered:: | Seres Therapeutics, Synlogic, Kaleido Biosciences, Finch Therapeutics, Vedanta Biosciences, Second Genome, Rebiotix, Enterome Bioscience, Assembly Biosciences, Axial Biotherapeutics, Evelo Biosciences, uBiome, Synlogic, Seres Therapeutics, Microbiome Therapeutics., Others. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis. |
Get more details on this report -
Driving Factors
The growing scientific understanding of the human microbiome’s impact on health and disease has opened new therapeutic possibilities. Advances in technologies like next-generation sequencing allow detailed analysis of microbial communities, enabling targeted treatments. The rising prevalence of chronic diseases such as Clostridioides difficile infection, inflammatory bowel disease, obesity, and diabetes has increased demand for innovative therapies. Additionally, the limitations and side effects of traditional treatments push patients and healthcare providers toward microbiome-based alternatives. Increased funding and research activities by both government agencies and private companies have accelerated product development. Growing awareness among healthcare professionals and patients about microbiome therapies’ benefits also supports market growth.
Restraining Factors
The high research and development costs, complex regulatory pathways, and limited understanding of microbiome variability among individuals. Challenges in clinical trial design, long approval timelines, and a lack of standardized manufacturing also hinder progress. Additionally, limited awareness among healthcare providers affects the adoption of microbiome-based treatments.
Market Segmentation
The United States microbiome therapeutics market share is classified into type and application.
- The FMT segment held the highest share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The USA microbiome therapeutics market is segmented by type into FMT and microbiome drugs. Among these, the FMT segment held the highest share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to its proven success in treating recurrent Clostridioides difficile infections, with success rates of 85–90%. Its established clinical use, growing demand, and improved delivery methods like capsules have strengthened its lead over microbiome drugs, which are still emerging in development.
- The C. difficile segment held a substantial share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The U.S. microbiome therapeutics market is segmented by application into C. difficile, Crohn’s disease, inflammatory bowel disease, diabetes, and others. Among these, the C. difficile segment held a substantial share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is attributed to the high success rate of Fecal Microbiota Transplantation (FMT) in treating recurrent infections. Its proven clinical effectiveness, FDA-approved therapies, and urgent medical need make it the leading application, surpassing areas like Crohn’s disease, inflammatory bowel disease, diabetes, and others.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the United States microbiome therapeutics market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Seres Therapeutics
- Synlogic
- Kaleido Biosciences
- Finch Therapeutics
- Vedanta Biosciences
- Second Genome
- Rebiotix
- Enterome Bioscience
- Assembly Biosciences
- Axial Biotherapeutics
- Evelo Biosciences
- uBiome
- Synlogic
- Seres Therapeutics
- Microbiome Therapeutics.
- Others
Recent Developments:
- In June 2023, Seres Therapeutics and Nestlé Health Science announced the U.S. commercial availability of VOWST, the first FDA-approved oral microbiota-based therapeutic to prevent recurrence of C. difficile infection, VOWST marked a significant advancement in microbiome therapeutics, offering a novel, non-antibiotic treatment option for patients at high risk of recurrent infection.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the USA, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the U.S. microbiome therapeutics market based on the below-mentioned segments:
United States Microbiome Therapeutics Market, By Type
- FMT
- Microbiome Drugs
United States Microbiome Therapeutics Market, By Application
- C. difficile
- Crohn’s disease
- Inflammatory Bowel Disease
- Diabetes
- Others
Need help to buy this report?