United States Medical Writing Market Size, Share, and COVID-19 Impact Analysis, By Type (Scientific Writing, Clinical Writing, Regulatory Writing, and Others), By Application (Medical Education, Medical Journalism, Medico Marketing, and Others), By End-User (Biotechnology Companies, Contract Research Organizations, and Others), and US Medical Writing Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareUSA Medical Writing Market Insights Forecasts to 2035
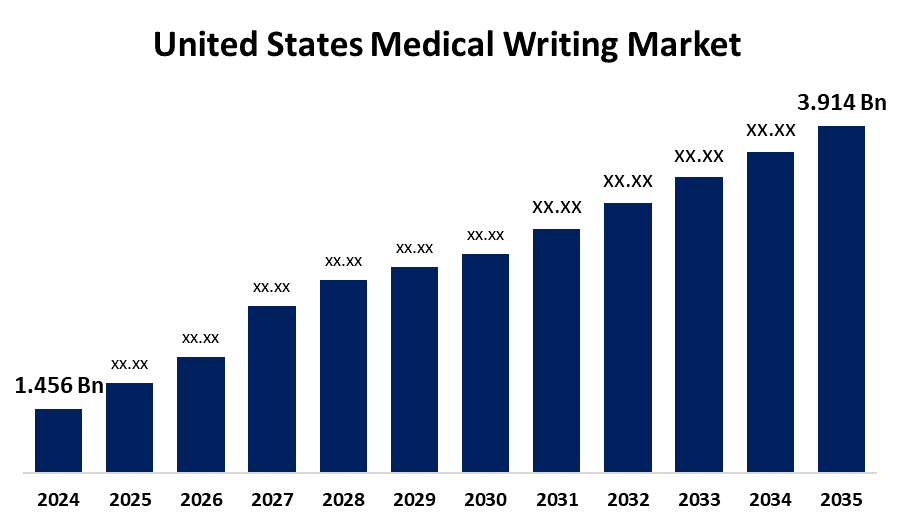
- The US Medical Writing Market Size was Estimated at USD 1.456 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 9.41% from 2025 to 2035
- The USA Medical Writing Market Size is Expected to Reach USD 3.914 Billion by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the US Medical Writing Market Size is Anticipated to Reach USD 3.914 Billion by 2035, Growing at a CAGR of 9.41% from 2025 to 2035. The market growth is driven by increasing research and development studies, rising regulatory submissions, and an increasing proportion of clinical trials.
Market Overview
The US medical writing market ensures industry compliance by providing specialized writing services for the biotechnology, pharmaceutical, and healthcare industries, including marketing materials, clinical trial reports, research papers, and regulatory documents. The field of medical writing is dedicated to producing organized scientific reports about healthcare and medicine. It entails creating a range of content, such as clinical trial reports, instructional materials, promotional literature, and regulatory submission documents. The focus on the complexities of healthcare, medications, biotechnology, and other medical topics makes medical writing distinct. It necessitates a thorough comprehension of medical terminology, adherence to legal requirements, and the capacity to communicate difficult ideas understandably. A drug's marketing from the lab to the market depends heavily on medical writing, which acts as a bridge connecting patients, healthcare providers, researchers, and regulatory bodies. It gives complex scientific data structure and clarity, converting it into actionable narratives that promote comprehension. Medical writing comes in a variety of forms, each with a specific function and target audience within the healthcare industry.
Report Coverage
This research report categorizes the market for the US medical writing market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the US medical writing market. Recent market developments and competitive strategies such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the US medical writing market.
United States Medical Writing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 1.456 Billion |
| Forecast Period: | 2024-2035 |
| Forecast Period CAGR 2024-2035 : | 9.41% |
| 2035 Value Projection: | USD 3.914 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 170 |
| Tables, Charts & Figures: | 150 |
| Segments covered: | By Type, By Application, By End-User, and COVID-19 Impact Analysis. |
| Companies covered:: | Quanticate, Trilogy Writing and Consulting GmbH, Parexel International Corporation, Freyr, LabCorp Drug Development, Cactus Communications, Omics International, IQVIA Holdings, Inc., Siro Clinpharm Private Limited, and Others. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Growing approval from regulatory authorities:
In the pharmaceutical and biopharmaceutical sectors, medical writing is becoming more and more significant as insurance companies and regulatory agencies require comprehensive details regarding product development processes. In addition to offering concise summaries of intricate data and recommendations for process enhancements, medical writers offer insightful commentary on protocol design. They can swiftly process and evaluate data following guidelines, which speeds up the development of new drugs. In addition to this, medical writing is becoming more and more important for getting regulatory approvals without any complications.
Rising trend for clinical trial documentation drives the market growth:
The rise of personalized medicine and biologics, which require accurate, customized documentation to satisfy regulatory requirements and support scientific communication, is anticipated to drive significant growth in the medical writing market. For medical writers with specific expertise in biologics, gene therapies, and cell-based treatments, the expansion of these treatments offers a substantial opportunity. Medical writing services in the USA are growing as a result of the growth of pharmaceutical companies and shifting healthcare regulations. There is an increasing need for writers who are multilingual and knowledgeable about local regulatory requirements as pharmaceutical companies conduct clinical trials in the US. Medical writers are experiencing an opportunity to produce content for eHealth and digital health platforms as a result of the healthcare sector's digital transformation.
Restraining Factors
The US medical writing market faces challenges due to strict regulatory requirements, high competition, a lack of subject matter expertise, increasing automation, and outsourcing challenges.
Market Segmentation
The USA medical writing market share is classified into type, application, and end-user.
- The clinical writing segment held the largest market share of 38.41% in 2024 and is expected to grow at a significant CAGR during the forecast period.
The US medical writing market is segmented by type into scientific writing, clinical writing, regulatory writing, and others. Among these, the clinical writing segment held the largest market share of 38.41% in 2024 and is expected to grow at a significant CAGR during the forecast period. This is attributed to the improved patient care, providing complex medical information in simple and concise medical information, and increasing productivity.
- The medical journalism segment accounted for the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The US medical writing market is segmented by application into medical education, medical journalism, medico marketing, and others. Among these, the medical journalism segment accounted for the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is driven by the rapid advancements and the growing significance of medical information, making skilled medical writers necessary. Medical journalism explores, reports, and disseminates medical issues to a wide audience.
- The contract research organizations segment accounted for the largest market share of 66.11% in 2024 and is expected to grow at a significant CAGR during the forecast period.
The US medical writing market is segmented by end-user into biotechnology companies, contract research organizations, and others. Among these, the contract research organizations segment accounted for the largest market share of 66.11% in 2024 and is expected to grow at a significant CAGR during the forecast period. The sector growth is attributed to the medical writing services, influenced by biotechnology investments, mergers, and product development. Some pharmaceutical companies are outsourcing drug development to CROs, enhancing research studies and efficiency.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the US medical writing market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Quanticate
- Trilogy Writing and Consulting GmbH
- Parexel International Corporation
- Freyr
- LabCorp Drug Development
- Cactus Communications
- Omics International
- IQVIA Holdings, Inc.
- Siro Clinpharm Private Limited
- Others
Recent Developments:
- In June 2024, Certara, Inc. introduced its next-generation CoAuthor regulatory writing software, designed for medical writers. It combines generative AI, document templates, Microsoft Word integration, and structured content authoring tools to accelerate regulatory document creation while maintaining a human-in-the-loop approach. Medical writing is a crucial part of drug development.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the United States, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the US medical writing market based on the below-mentioned segments:
US Medical Writing Market, By Type
- Scientific Writing
- Clinical Writing
- Regulatory Writing
- Others
US Medical Writing Market, By Application
- Medical Education
- Medical Journalism
- Medico Marketing
- Others
US Medical Writing Market, By End-User
- Biotechnology Companies
- Contract Research Organizations
- Others
Need help to buy this report?