United States Cardiac Troponin Market Size, Share, and COVID-19 Impact Analysis, By Type (Troponin I and Troponin T), By Indication (Acute Coronary Syndrome, Myocardial Infarction, Congestive Heart Failure, and Others), and US Cardiac Troponin Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareUSA Cardiac Troponin Market Insights Forecasts to 2035
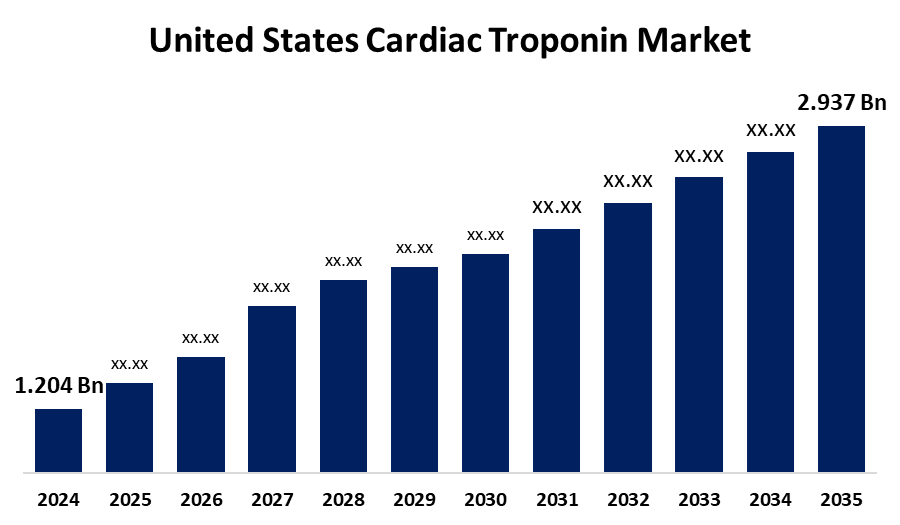
- The US Cardiac Troponin Market Size was Estimated at USD 1.204 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 8.44% from 2025 to 2035
- The USA Cardiac Troponin Market Size is Expected to reach USD 2.937 Billion by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the US Cardiac Troponin Market Size is anticipated to reach USD 2.937 Billion by 2035, growing at a CAGR of 8.44% from 2025 to 2035. The market growth is attributed to the increasing prevalence of cardiovascular disease, a rising proportion of the geriatric population, and technological advancements in medications.
Market Overview
The production, distribution, and usage of cardiac troponin tests—essential biomarkers for identifying heart muscle damage and diagnosing diseases such as myocardial infarction, acute coronary syndrome, and congestive heart failure are the main objectives of the US cardiac troponin market. An extremely sensitive and specific protein called cardiac troponin is utilized to diagnose acute myocardial infarction. It is a recommended biomarker for acute coronary syndrome (ACS) and is released following myocardial cell damage. The cardiac regulatory proteins troponin I (cTnl) and troponin T (cTnT) are involved in regulating the calcium-mediated interaction between actin and myosin. They are a recommended biomarker for identifying myocardial infarction because of their excellent cardiac specificity. Troponin levels that rise or decline can be used to distinguish between acute myocardial infarction and chronic heart disease. The market is expanding due to the rising incidence of cardiovascular disorders and technological developments. Future market expansion is also anticipated to be fueled by the use of point-of-care testing in hospital emergency rooms.
Report Coverage
This research report categorizes the market for the US cardiac troponin market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the US cardiac troponin market. Recent market developments and competitive strategies such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the US cardiac troponin market.
United States Cardiac Troponin Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 1.204 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 8.44% |
| 2035 Value Projection: | USD 2.937 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 173 |
| Tables, Charts & Figures: | 117 |
| Segments covered: | By Type, By Indication and COVID-19 Impact Analysis |
| Companies covered:: | Abbott Laboratories, PerkinElmer Inc., Beckman Coulter, Inc., LifeSign LLC., Others, and key vendors |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving factors
The rising prevalence of cardiovascular diseases (CVDs), which are frequently associated with acute myocardial infarction (AMI), is driving an upsurge in the troponin sector. Troponin tests are essential for the early identification and treatment of individuals with suspected AMI because they have a high sensitivity and specificity for detecting myocardial injury. High-sensitivity assays have been created as a result of technological developments in troponin testing, improving clinical outcomes and diagnostic precision. Point-of-care testing (POCT), which enables rapid and easy testing of biomarkers like troponin at the patient's bedside or in ambulatory settings, is driving the market's growth. In emergency departments, critical care units, and outpatient clinics, where timely diagnosis and treatment decisions are essential, this is especially significant.
Restraining Factors
The high cost of advanced diagnostic equipment, regulatory hurdles, the need for skilled workers, intense competition among major players, limited accessibility in some areas, restrictions on point-of-care testing, and challenges for smaller businesses trying to enter may hinder market growth.
Market Segmentation
The USA cardiac troponin market share is classified into type, indication, and end-user.
- The troponin I segment held the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The US Cardiac Troponin market is segmented by product into troponin I and troponin T. Among these, the troponin I segment held the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is attributed to the actionable, reliable results, highly sensitive, and improved diagnostic clarity.
- The acute coronary syndrome segment accounted for the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The US cardiac troponin market is segmented by indication into acute coronary syndrome, myocardial infarction, congestive heart failure, and others. Among these, the acute coronary syndrome segment accounted for the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period. The increasing prevalence of coronary diseases, reduced blood flow, cholesterol deposition, and an increasing number of the geriatric population.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the US cardiac troponin market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott Laboratories
- PerkinElmer Inc.
- Beckman Coulter, Inc.
- LifeSign LLC.
- Others
Recent Developments:
- In October 2024, Siemens Healthineers received FDA clearance for its Atellica IM high-sensitivity troponin I (TnIH) test, which can help healthcare providers identify patients at risk of death and major cardiac events up to 1 year after presenting with acute coronary syndrome symptoms.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the United States, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the US cardiac troponin market based on the below-mentioned segments:
US Cardiac Troponin Market, By Type
- Troponin I
- Troponin T
US Cardiac Troponin Market, By Indication
- Acute Coronary Syndrome
- Myocardial Infarction
- Congestive Heart Failure
- Others
Need help to buy this report?