Unites States C-Reactive Protein Testing Market Size, Share, and COVID-19 Impact Analysis, By Assay Type (Immuno-turbidimetric Assay, ELISA, Clinical, Non-clinical, Chemiluminescence Immunoassay, and Others), By End-Use (Clinics, Hospitals, Laboratories, Assisted Living Healthcare Facilities, Home Care Settings, and Others), and United States C-Reactive Protein Testing Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareUnited States C-Reactive Protein Testing Market Insights Forecasts to 2035
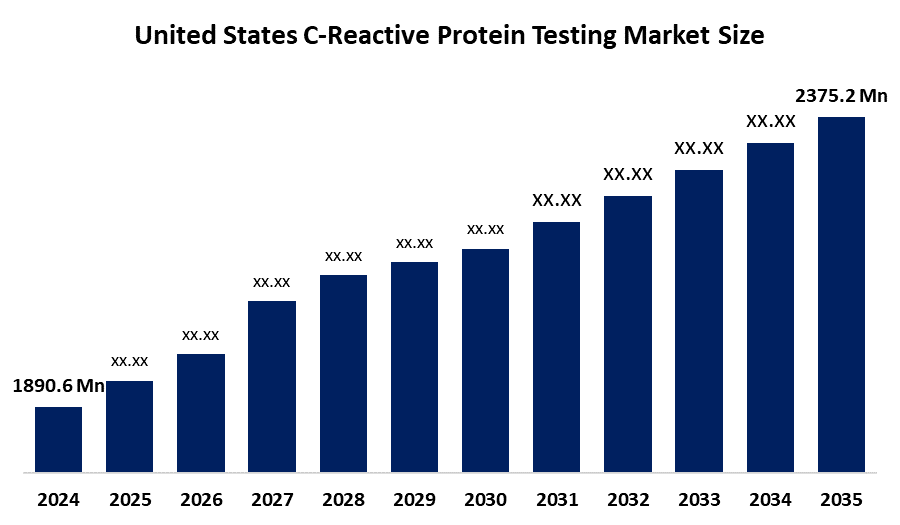
- The United States C-Reactive Protein Testing Market Size Was Estimated at USD 1890.6 Million in 2024
- The Market Size is Expected to Grow at a CAGR of Around 2.1% from 2025 to 2035
- The United States C-Reactive Protein Testing Market Size is Expected to Reach USD 2375.2 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the United States C-reactive protein testing market is anticipated to reach USD 2375.2 million by 2035, growing at a CAGR of 2.1% from 2025 to 2035. The United States -reactive protein testing market is driven by the rising demand for precision medicine, which uses CRP testing to tailor treatments to individual patients, and the increasing aging population, along with the prevalence of cardiovascular disorders, creating higher demand for inflammation and risk assessment diagnostics.
Market Overview
The United States C-reactive protein (CRP) testing market is the healthcare diagnostics market in the United States that involves the production, sale, and use of tests that measure CRP levels in blood to detect inflammation and assess disease risk. The United States CRP testing market is applied in hospitals, diagnostic labs, clinics, and cardiology centers for inflammation detection, cardiovascular risk assessment, treatment monitoring, research, and emerging home testing solutions. The market is growing due to the rising demand for precision medicine, which uses CRP testing to tailor treatments to individual patients, and the increasing aging population, along with the prevalence of cardiovascular disorders, creating higher demand for inflammation and risk assessment diagnostics.
Key opportunities in the market include expanding point-of-care testing in primary care, integrating tests with digital health platforms, and advanced, rapid testing solutions in emergency medicine. In December 2025, Serial C-Reactive Protein Point-of-Care testing to optimize antibiotic treatment in hospitalized children with signs of infection in Zanzibar. A feasibility study was published, showing that serial CRP POCT can be safely integrated into hospital workflows and reduce antibiotic treatment days in neonates and children compared with standard care.
Report Coverage
This research report categorizes the market for the United States C-reactive protein testing market based on various segments and regions, and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the United States C-reactive protein testing market. Recent market developments and competitive strategies, such as expansion, product launch, development, partnership, merger, and acquisition, have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the United States C-reactive protein testing market.
United States C-Reactive Protein Testing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 1890.6 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR Of 2.1% |
| 2035 Value Projection: | USD 2375.2 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 220 |
| Tables, Charts & Figures: | 138 |
| Segments covered: | By Assay Type,By End-Use |
| Companies covered:: | Boditech Med, F. Hoffmann-La Roche Ltd, Getein Biotech, HORIBA Ltd, Abbot Laboratories, Thermo Fisher Scientific inc., Merck KGaA, Quest Diagnostics Incorporated, Randox Laboratories, Siemens Healthineers,and Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The C-reactive protein (CRP) testing market in the United States is driven by increasing healthcare expenditure, which is significantly propelling the CRP testing market, as greater funding allows healthcare facilities to enhance diagnostic infrastructure and broaden testing services. The expanding focus on precision medicine is driving higher demand for CRP testing, increasing awareness of inflammation related condition, technological advancements in diagnosis, and emphasis on preventive healthcare, driving the United States C-reactive protein testing market.
Restraining Factors
The C-reactive protein testing market in the United States is mostly constrained by high diagnostic costs, limited insurance coverage, low awareness in some regions, competition from alternative biomarkers, and strict regulatory requirements, all of which hinder widespread adoption across healthcare facilities.
Market Segmentation
The United States C-reactive protein testing market is classified into assay type and end-use.
- The immuno-turbidimetric assay segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The United States C-reactive protein testing market is segmented by assay type into immune-turbidimetric assay, ELISA, clinical, non-clinical, chemiluminescence immunoassay, and others. Among these, the immuno-turbidimetric assay segment accounted for the largest revenue market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to its extensive use in hospitals and clinical laboratories, healthcare providers favor this method for its accuracy, fast results, and cost-efficiency compared to alternative testing techniques. The rising incidence of cardiovascular diseases, diabetes, and inflammatory conditions in the United States has driven greater demand for routine CRP testing, with immuno-turbidimetric assays becoming the preferred option for large-scale diagnostic screening.
- The hospitals segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period.
The United States C-reactive protein testing market is segmented by end-use into clinics, hospitals, laboratories, assisted living healthcare facilities, home care settings, and others. Among these, the hospitals segment dominated the share in 2024 and is anticipated to grow at a remarkable CAGR during the forecast period. This is due to Hospitals remain the primary drivers of the CRP testing market, serving as key centers for diagnosing and managing acute and chronic conditions. Physicians utilize CRP tests to monitor infections, cardiovascular complications, and inflammatory diseases, supported by advanced diagnostic infrastructure and integration with high-throughput analyzers.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the United States C-reactive protein testing market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Boditech Med
- F. Hoffmann-La Roche Ltd
- Getein Biotech
- HORIBA Ltd
- Abbot Laboratories
- Thermo Fisher Scientific inc.
- Merck KGaA
- Quest Diagnostics Incorporated
- Randox Laboratories
- Siemens Healthineers
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments:
- In January 2026, HORIBA Ltd obtained CE VDR certification for the new Yumizen H500 CRP benchtop hematology analyzer, designed for simultaneous 5-part differential (DIFF) and rapid CRP measurement, with rollout planned for various markets.
- In November 2025, Boditech Med introduced the ichromaTM 50 plus and other next- generation POC platforms at MEDICA2025.
Market Segment
This study forecasts revenue at the United States, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the United States C-reactive protein testing market based on the below-mentioned segments:
United States C-Reactive Protein Testing Market, By Assay Type
- Immuno-turbidimetric Assay
- ELISA
- Clinical
- Non-clinical
- Chemiluminescence Immunoassay
- Others
United States C-Reactive Protein Testing Market, By End-Use
- Clinics
- Hospitals
- Laboratories
- Assisted Living Healthcare Facilities
- Home Care Settings
- Others
Need help to buy this report?