United Kingdom Preclinical CRO Market Size, Share, and COVID-19 Impact Analysis, By Service (Bioanalysis and DMPK studies, Toxicology Testing, Compound Management, Chemistry, Safety Pharmacology, Others), By Model (Patient-Derived Organoid (PDO) Model, Patient-Derived Xenograft Model), and United Kingdom Preclinical CRO Market Insights, Industry Trend, Forecasts to 2035
Industry: Healthcarenited Kingdom Preclinical CRO Market Insights Forecasts to 2035
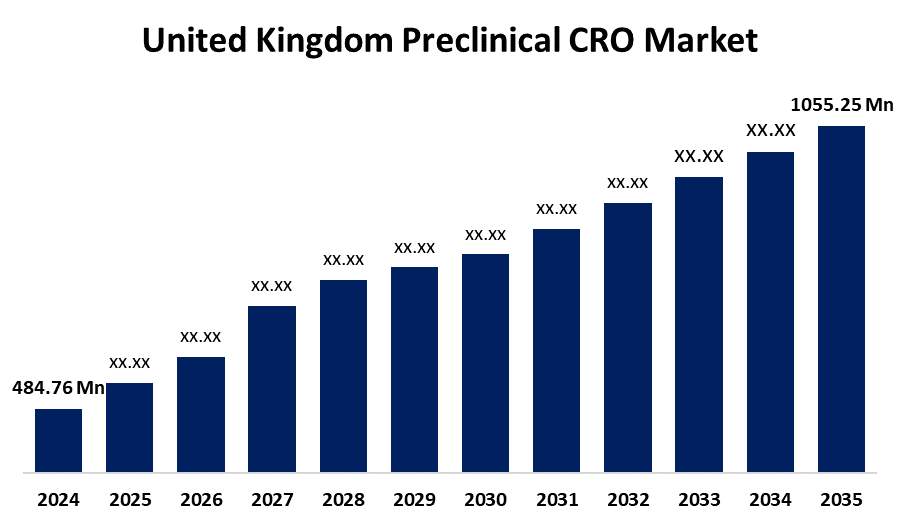
- The United Kingdom Preclinical CRO Market Size was estimated at USD 484.76 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 7.33% from 2025 to 2035
- The United Kingdom Preclinical CRO Market Size is Expected to Reach USD 1055.25 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the United Kingdom Preclinical CRO Market is anticipated to reach USD 1055.25 million by 2035, growing at a CAGR of 7.33% from 2025 to 2035. Major drivers driving the market in the region include the increasing focus on core competencies among organizations, the growing complexity of the regulatory environment, the expanding expenditure on research and development (R&D) efforts, and recent developments in specialized therapies.
Market Overview
The United Kingdom preclinical CRO market refers to the industry focused on the production and application of services that are increasingly utilized by biotechnology and biopharmaceutical companies to manage their R&D efforts for new treatments. The outsourcing of research studies to preclinical CROs by healthcare organizations and research institutes has been fueled by factors including improved preclinical testing, decentralized studies, regulatory compliance, risk management, and cost reduction. There are numerous local businesses, which contribute to the market's modest fragmentation. These market participants are using tactics including partnerships, acquisitions, and mergers in an effort to increase their market share. Additionally, businesses are spending money on creating better products. Additionally, they are concentrating on keeping their prices competitive. Growing preclinical CRO outsourcing and early research activities in the drug development process are the main drivers of the preclinical CRO market. Finding the precise target group and improving enrollment can be greatly aided by multicenter Proof-Of-Concept (POC), First-In-Human (FIH), and preclinical trials.
Report Coverage
This research report categorizes the market for the United Kingdom preclinical CRO market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the United Kingdom preclinical CRO market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the United Kingdom preclinical CRO market.
United Kingdom Preclinical CRO Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 484.76 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 7.33% |
| 2035 Value Projection: | USD 1055.25 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 210 |
| Tables, Charts & Figures: | 112 |
| Segments covered: | By Service, By Model and COVID-19 Impact Analysis |
| Companies covered:: | Sygnature Discovery, CRO-Solutions, Paracelsis Ltd, Pharmaron, Gifford Bioscience, Neurosolutions, Veristat, BioServUK Ltd, Others. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The market for the United Kingdom preclinical CRO is driven by the increased demand for preclinical contract research organization (CRO) services brought on by increased R&D expenditure for drug development. CRO's specialized knowledge, cutting-edge technology, and more inclusive preclinical testing services have become increasingly important to pharmaceutical and biotechnological enterprises. In addition, more regulatory criteria that must be met, increased investments in novel medicines, and more thorough biopharmaceutical research are driving market expansion. Additionally, the need for preclinical CRO services has grown as a result of the rising incidence of chronic illnesses and the accelerated rate of drug development. Preclinical CRO market growth is also being pushed by ongoing technical advancements like AI-driven drug discovery and improved laboratory work, which make CRO partnerships more effective and appealing. A major factor driving the need for new treatments and preclinical trial activity is the increasing prevalence of chronic illnesses like cancer, heart disease, and neurological disorders.
Restraining Factors
The market expansion could be hampered by strict regulatory requirements for preclinical CRO services, standards, and high-end testing, such as genotoxicity, which are also carried out in-house by pharmaceutical companies due to regulatory rules.
Market Segmentation
The United Kingdom preclinical CRO market share is classified into service, and model.
- The toxicology testing segment held a significant share in 2024 and is anticipated to grow at a rapid pace during the forecast period.
The United Kingdom preclinical CRO market is segmented by service into bioanalysis and DMPK studies, toxicology testing, compound management, chemistry, safety pharmacology, and others. Among these, the toxicology testing segment held a significant share in 2024 and is expected to grow at a rapid pace during the forecast period. The widespread usage focuses on assessing the safety and possible side effects of novel drug candidates; this section is essential. Its vital function in guaranteeing drug safety before it enters clinical trials accounts for its market supremacy.
- The patient-derived organoid (PDO) model segment held the largest market share in 2024 and is anticipated to grow at a significant CAGR during the forecast period.
The United Kingdom preclinical CRO market is segmented by model into patient-derived organoid (PDO) model, and patient-derived xenograft model. Among these, the patient-derived organoid (PDO) model segment held a significant share in 2024 and is anticipated to grow at a significant CAGR during the forecast period. This is influenced by its sophisticated ability to precisely duplicate the architecture of human tissue and forecast the therapeutic reactions of individual patients. PDOs improve therapeutic efficacy and safety prediction by offering a more accurate depiction of human biology, which gives them a major edge over traditional models.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the United Kingdom preclinical CRO market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Sygnature Discovery
- CRO-Solutions
- Paracelsis Ltd
- Pharmaron
- Gifford Bioscience
- Neurosolutions
- Veristat
- BioServUK Ltd
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at United Kingdom, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the United Kingdom preclinical CRO market based on the below-mentioned segments:
United Kingdom Preclinical CRO Market, By Service
- Bioanalysis and DMPK studies
- Toxicology Testing
- Compound Management
- Chemistry
- Safety Pharmacology
- Others
United Kingdom Preclinical CRO Market, By Model
- Patient-Derived Organoid (PDO) Model
- Patient-Derived Xenograft Model
Need help to buy this report?