United Kingdom Orphan Diseases Drugs Market Size, Share, and COVID-19 Impact Analysis, By Therapy Area (Oncology, Hematology, Neurology, Endocrinology, Cardiovascular, Respiratory, Immunotherapy, and Others), By Drug Type (Biologics and Non-Biologics), and United Kingdom Orphan Diseases Drugs Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareUnited Kingdom Orphan Diseases Drugs Market Size Insights Forecasts to 2035
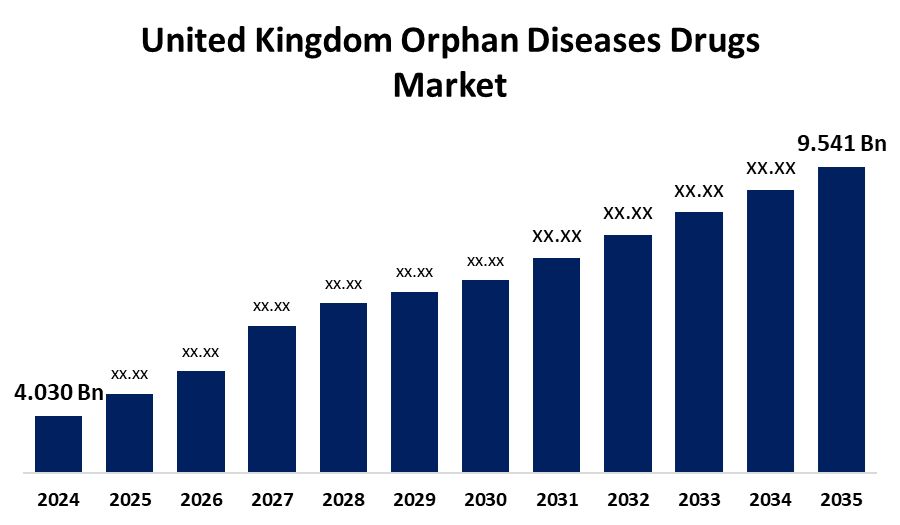
- The United Kingdom Orphan Diseases Drugs Market Size was Estimated at USD 4.030 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 8.15% from 2025 to 2035
- The United Kingdom Orphan Diseases Drugs Market Size is Expected to Reach USD 9.541 Billion by 2035
Get more details on this report -
According to a Research Report Published by Spherical Insights and Consulting, the United Kingdom Orphan Diseases Drugs Market Size is anticipated to reach USD 9.541 Billion by 2035, growing at a CAGR of 8.15% from 2025 to 2035. There has been a noticeable increase in the general public's knowledge and attention regarding rare diseases, and demand is further promising and effective treatments for rare medical situations has boosted the market growth.
Market Overview
The United Kingdom Orphan Diseases Drugs Market Size refers to the industry focused on the production and application of pharmaceutical products used for the diagnosis, prevention, and treatment of uncommon medical disorders. They are manufactured to fulfill a particular public need, these drugs usually have a small market because they are produced for a specific patient population. The growth of this market is also expected to be supported by rising healthcare costs and the incidence of uncommon diseases. The market for orphan drugs in the UK is expanding as a result of higher R&D expenditures and legal incentives for creating rare disease cures. The strong medical need in the orphan disease arena, the rising incidence of rare illnesses, and the development of innovative technologies for their diagnosis and treatment are all factors contributing to the market's anticipated continued expansion. The growth of the orphan medication industry is also expected to be fueled by advantageous government policies and reimbursement guidelines. For instance, the Medicines and Healthcare products Regulatory Agency (MHRA) approved leniolisib phosphate (Joenja) as the first medicine for activated phosphoinositide 3-kinase delta syndrome (APDS), a rare immune disease. APDS was an inherited disorder that weakened the immune system, making patients more vulnerable to repeated lung infections and increasing their risk of developing blood cell cancers like lymphoma. The condition often presents symptoms within the first two years of life, including failure to grow and develop normally.
Report Coverage
This research report categorizes the market for the United Kingdom orphan diseases drugs market based on various segments and regions, and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the United Kingdom orphan diseases drugs market. Recent market developments and competitive strategies such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the United Kingdom orphan diseases drugs market.
United Kingdom Orphan Diseases Drugs Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 4.030 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 8.15% |
| 2035 Value Projection: | USD 9.541 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 184 |
| Tables, Charts & Figures: | 115 |
| Segments covered: | By Therapy Area, By Drug Type and COVID-19 Impact Analysis |
| Companies covered:: | Octagenix, Quoin Pharmaceuticals Ltd, Amphista Therapeutics, GlaxoSmithKline, AstraZeneca, Immunocore, Others, and key vendors |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The market for the United Kingdom orphan diseases drugs is primarily driven by the prevalence of age-related uncommon disorders, such as neurological ailments, and the increasing need for specialist therapies. Furthermore, the development of more potent orphan medications is being made possible by developments in biotechnology and medical research. Additionally, demand is being driven in part by the increased activism and awareness of rare diseases. There is growing demand for improved access to therapies as patient organizations and advocacy groups bring attention to the needs of people with uncommon disorders.
Restraining Factors
However, the services for screening, slow therapy interruptions, limited operational procedures that keep away small and medium-sized clinics, hospitals, and ambulatory services, and inconsistent resources for academic and research institutions and major academic institutions are the barriers that prohibit the market expansion.
Market Segmentation
The United Kingdom orphan diseases drugs market share is classified into therapy area and drug type.
- The oncology segment held the highest share in 2024 and is anticipated to grow at a substantial CAGR over the forecast period.
The United Kingdom orphan diseases drugs market is segmented by therapy area into oncology, hematology, neurology, endocrinology, cardiovascular, respiratory, immunotherapy, and others. Among these, the oncology segment held the highest share in 2024 and is anticipated to grow at a substantial CAGR over the forecast period. Significant developments in cancer treatments, including gene therapies, immunotherapies, and targeted treatments, are fueling the segment's expansion. It is anticipated that these developments, along with robust regulatory backing and a growing emphasis on uncommon tumors, will keep driving the oncology segment's expansion.
- The biologics segment held the highest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The United Kingdom orphan diseases drugs market is segmented by drug type into biologics and non-biologics. Among these, the biologics segment held the highest share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is because a broad category of medications includes items made from human blood and plasma, growth factors, monoclonal antibodies, vaccinations, and immune modulators. The biological orphan pharmaceuticals area is dominated by a growing number of biological products offered by significant market players.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the United Kingdom orphan diseases drugs market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Octagenix
- Quoin Pharmaceuticals Ltd
- Amphista Therapeutics
- GlaxoSmithKline
- AstraZeneca
- Immunocore
- Others
Recent Developments:
- In April 2024, LifeArc launched four new Translational Centres for Rare Diseases, backed by a £40 million investment, to accelerate the development of new tests, treatments, and potential cures for people living with rare diseases. These virtual research centres focused on areas with significant unmet medical needs, tackling barriers that often prevented new treatments from reaching patients. The initiative aimed to speed up rare disease clinical trials and foster collaboration among leading scientists and clinical specialists across the UK.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at United Kingdom, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the United Kingdom orphan diseases drugs market based on the below-mentioned segments
United Kingdom Orphan Diseases Drugs Market, By Therapeutic Area
- Oncology
- Hematology
- Neurology
- Endocrinology
- Cardiovascular
- Respiratory
- Immunotherapy
- Others
United Kingdom Orphan Diseases Drugs Market, By Drug Type
- Biologics
- Non-Biologics
Need help to buy this report?