United Kingdom Clinical Trials Market Size, Share, and COVID-19 Impact Analysis, By Phase (Phase I, Phase II, Phase III, Phase IV), By Study Design (Interventional, Observational, Expanded access), and United Kingdom Clinical Trials Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareUnited Kingdom Clinical Trials Market Insights Forecasts to 2035
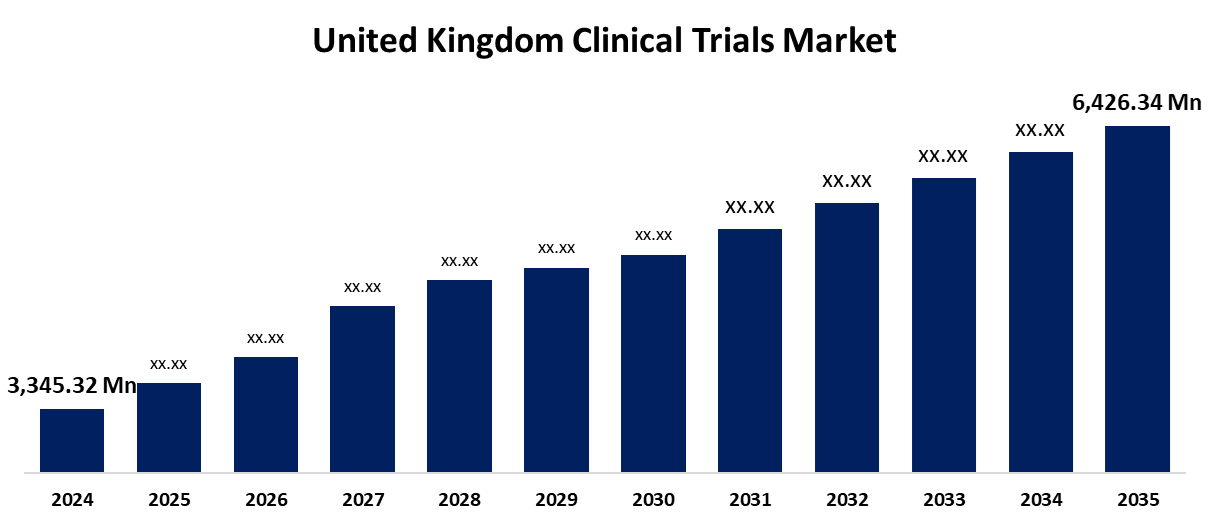
- The United Kingdom Clinical Trials Market Size Was Estimated at USD 3,345.32 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 6.11% from 2025 to 2035
- The United Kingdom Clinical Trials Market Size is Expected to Reach USD 6,426.34 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the United Kingdom Clinical Trials Market is anticipated to reach USD 6,426.34 million by 2035, growing at a CAGR of 6.11% from 2025 to 2035. Strong government backing and increasing regional awareness of rare illnesses and healthy economic resources are driving the United Kingdom clinical trials market's expansion.
Market Overview
The United Kingdom clinical trials market refers to the business that supports the healthcare industry by focusing on the research and testing of new medical treatments, drugs, and therapies on human participants before they receive regulatory approval. It encompasses pharmaceutical companies, contract research organizations (CROs), hospitals, and research institutions that conduct trials to evaluate the safety, efficacy, and side effects of medical interventions. Moreover, to guarantee participant safety and scientific correctness, clinical studies are regulated by authorities and adhere to stringent regulations. These trials, which frequently grant participants access to modern facilities and treatments, depend on the voluntary participation of those fulfill specific eligibility conditions. Growing emphasis on precision and customized treatment, particularly for chronic and complicated diseases, is the main driver clinical trial market's expansion. Some key market developments, such as in April 2025, a new analysis by the Medicines and Healthcare products Regulatory Agency (MHRA) and the University of Liverpool highlights opportunities to enhance UK clinical trials. The report, published in the British Journal of Clinical Pharmacology, finds that while the UK is a global leader in clinical research, there are areas for improvement, such as diversifying patient representation and expanding research into underrepresented conditions, which propels the market growth.
Report Coverage
This research report categorizes the market for the United Kingdom clinical trials market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the United Kingdom clinical trials market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the United Kingdom clinical trials market.
United Kingdom Clinical Trials Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 3,345.32 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 6.11% |
| 2035 Value Projection: | USD 6,426.34 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 210 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Phase, By Study Design and COVID-19 Impact Analysis |
| Companies covered:: | XARlabs, Alcyomics, TrialeX, iðun million steps, Care Across and others key players. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The market for United Kingdom clinical trials is driven by the rising need for novel drugs and substantial investment from several pharmaceutical, biopharmaceutical, and medical device businesses. Additionally, the market is anticipated to grow favorably due to the rising prevalence of chronic diseases, the globalization of clinical trials, the penetration of customized medicine, the quick advancement of technology, and the increased need for CROs to perform research. Furthermore, using novel technologies, including Electronic Data Capture (EDC), helps businesses manage patient data and streamline data gathering, which minimizes monitoring costs and speeds up clinical trial deadlines by allowing real-time data access and analysis, thus fueling the market growth.
Restraining Factors
The clinical trial industry in the UK continues to face substantial obstacles in terms of recruiting and retaining patients, which impedes the speed and success of medication development. Moreover, high dropout rates and delays from regulatory authorities in trials hamper the market expansion.
Market Segmentation
The United Kingdom clinical trials market share is classified into phase, and study design.
- The phase III segment accounted for the highest share in 2024 and is expected to grow at a significant CAGR over the forecast period.
The United Kingdom clinical trials market is divided by phase into phase I, phase II, phase III, and phase IV. Among these, the phase III segment accounted for the highest share in 2024 and is expected to grow at a significant CAGR over the forecast period. The segment growth is driven by assessing the long-term safety and efficacy of sophisticated treatments for diseases. Moreover, regulatory incentives supporting research and development might be potentially responsible for more late-stage studies and collaboration with specialized research centers. Further, the requirement for substantial clinical evidence to back up regulatory approvals has reinforced this segment's dominance in the market for clinical studies.
- The observational segment held a significant share in 2024 and is expected to grow at a rapid pace over the forecast period.
The United Kingdom clinical trials market is segmented by study design into interventional, observational, and expanded access. Among these, the observational segment held a significant share in 2024 and is expected to grow at a rapid pace over the forecast period. This is because they provide valuable insights into actual patient experiences, treatment outcomes, and illness patterns. These studies, which typically include observing people in their natural environments without intervention, allow researchers to get data on a range of topics related to health and illness. Further, observational studies examine delivery systems, resource utilization, and healthcare disparities.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the United Kingdom clinical trials market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- XARlabs
- Alcyomics
- TrialeX
- iðun million steps
- Care Across
- Others
Recent Developments:
- In May 2025, UK researchers launched clinical trials for a new African swine fever (ASF) vaccine at The Pirbright Institute, in partnership with The Vaccine Group (TVG). The vaccine is designed to offer a safer alternative to current live attenuated options.
- In February 2025, the UK Government launched the AI EDITH trial (Early Detection using Information Technology in Health) to improve early breast cancer detection. This initiative has part of the UK’s broader 10-Year Health Plan, aiming to modernize cancer care and enhance screening efficiency.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at United Kingdom, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the United Kingdom clinical trials market based on the below-mentioned segments:
United Kingdom Clinical Trials Market, By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
United Kingdom Clinical Trials Market, By Study Design
- Interventional
- Observational
- Expanded access
Need help to buy this report?