Global Specimen Validity Testing (SVT) Market Size, Share, and COVID-19 Impact Analysis, By Product and Services (Product {Assay Kits, Reagents & Calibrators, Disposables}, Service), By Type (Laboratory Testing and Rapid/POC Testing), By End User (Workplace, Drug Screening, Pain Management, & Drug Rehabilitation Centers), By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2022 - 2032.
Industry: HealthcareGlobal Specimen Validity Testing Market Insights Forecasts to 2032
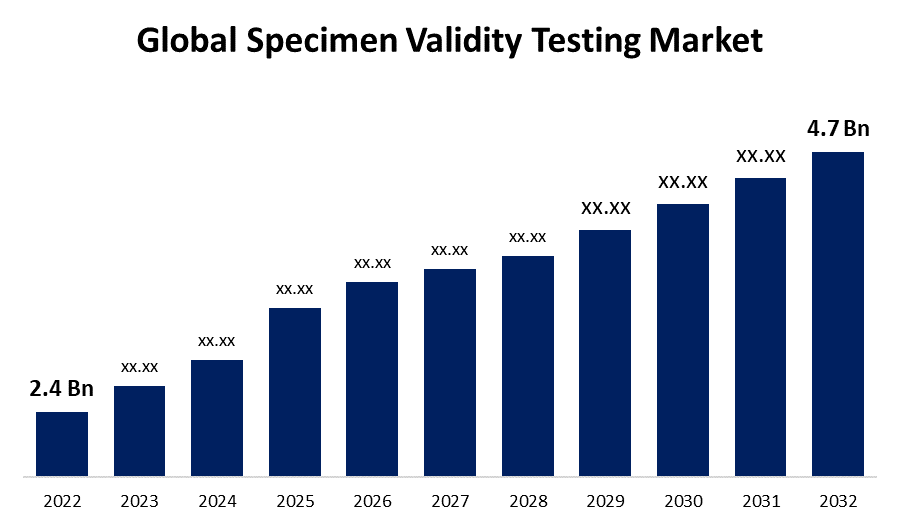
- The Global Specimen Validity Testing (SVT) Market Size was valued at USD 2.4 Billion in 2022.
- The Market Size is Growing at a CAGR of 6.9% from 2022 to 2032.
- The Worldwide Specimen Validity Testing (SVT) Market size is expected to reach USD 4.7 Billion by 2032.
- Asia Pacific is expected To Grow the fastest during the forecast period.
Get more details on this report -
The Global Specimen Validity Testing Market (SVT) Size is expected to reach USD 4.7 Billion by 2032, at a CAGR of 6.9% during the forecast period 2022 to 2032.
Market Overview
Specimen validity testing (SVT) is a technique for verifying the integrity and authenticity of biological samples, mostly urine, in several testing situations, including drug testing. The purpose of this testing is to ensure that the supplied sample has not been tainted, replaced, diluted, or tampered with in any manner that might affect the test findings. However, the high cost of specimen validity testing may pose a substantial challenge to market expansion in the coming years. Specimen validity testing (SVT) may incur additional expenditures in addition to normal drug testing processes. To effectively assess the integrity of materials, these tests frequently necessitate the use of specialized equipment and reagents. Instruments for monitoring temperature, pH, and specific gravity, as well as chemical reagents for identifying adulterants or masking agents, may be included. The expense of acquiring, maintaining, and calibrating such equipment, as well as the procurement of specific reagents, can all add to the overall cost of specimen validity testing.
Report Coverage
This research report categorizes the global specimen validity testing market based on various segments and regions and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global specimen validity testing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global specimen validity testing market. Technological innovation and advancement will further optimize the performance of the product, enabling it to acquire a wider range of applications in the downstream market.
Global Specimen Validity Testing (SVT) Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2022 |
| Market Size in 2022: | USD 2.4 Billion |
| Forecast Period: | 2022-2032 |
| Forecast Period CAGR 2022-2032 : | 6.9% |
| 2032 Value Projection: | USD 4.7 Billion |
| Historical Data for: | 2018-2021 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Product and Services, By Type, By End User, By Region. |
| Companies covered:: | Thermo Fisher, Sciteck, American Bio Medica Corporation, Alere, Express Diagnostics, Premier Biotech., Quest Diagnostics, Alere Toxicology, ACM Global Laboratories, Clinical Reference Laboratory (CRL), SureHire, and CannAmm |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Increased drug screening laboratories and diagnostic centers, together with better testing techniques, are some significant aspects projected to boost the market, resulting in the prevalence of high substances globally. Furthermore, the use of illegal substances such as marijuana, heroin, cocaine, hallucinogens, and other non-medical alcoholic fluids and psychotropic medications is raising public awareness of the global specimen validity testing sector. The government is also promoting drug screening programs in the workplace for the benefit of the public, which will drive revenue growth in the specimen validity testing market during the projected period. Moreover, SVT is used by law enforcement authorities to safeguard offenders and suspects by detecting the presence and traces of illegal narcotics. It aids law enforcement authorities in researching the behavior and reasons for a certain crime. The global specimen validity testing industry is being driven by an increase in illicit drug usage. Furthermore, strict testing regulations and government funding to combat drug addiction are projected to boost the specimen validity testing market globally during the forecast period.
Restraining Factors
The availability of various quick drug screening tests on the market that yield reliable outcomes might hinder the deployment of specimen validity testing. Regulatory clearance processes for Rapid Test products are likely to be time-consuming and expensive over the projection period, posing a hindrance to market expansion. Furthermore, specimen validity testing is not harmonized with workplace drug testing protocols and point-of-care testing, which is projected to limit its adoption and, to some degree, restrict market growth during the projection period.
Market Segmentation
- In 2022, the product segment is dominating the market with the largest market share during the forecast period.
Based on the product and services, the global specimen validity testing market is segmented into products (assay kits, reagents, and calibrators, disposables), services. Among these segments, the product (assay kits, reagents, calibrators, disposables) segment is dominating the market with the largest revenue share during the forecast period due to various benefits provided by goods in the early and precise detection of specimens and samples. These products are intended to identify different types of sample tampering or adulteration, such as dilution, substitution, or the addition of adulterants or masking agents. SVT products increase drug testing accuracy while inhibiting sample manipulation or adulteration.
- In 2022, the laboratory testing segment is influencing the largest market share during the forecast period.
Based on the type, the global specimen validity testing market is bifurcated into different categories such as laboratory testing and rapid/POC testing. Among these segments, the laboratory testing segment is dominating the market due to the many clinical benefits provided by laboratory testing for sample and specimen detection. These laboratories are outfitted with cutting-edge technology and equipment, as well as highly skilled and well-trained personnel. Furthermore, laboratory testing enables a thorough examination of biological samples to determine their integrity and validity. This thorough examination aids in spotting any irregularities or discrepancies in the samples, providing accurate and trustworthy drug test results. Furthermore, greater accuracy and dependability, expert interpretation and consultation, as well as research and development efforts, are likely to boost market expansion in the forecast period.
- In 2022, the drug screening segment is influencing the largest market share during the forecast period.
Based on the end user, the global specimen validity testing market is classified into workplace, drug screening, pain management, & drug rehabilitation centers. Among these segments, drug screening is dominating the market due to the availability of modern detection and screening technology and services to accommodate a diverse consumer base. The market is predicted to develop due to improved operability and accessibility to a full range of tests involving the identification of illegal drug usage. The growing incidence of adulteration and tampering with urine samples for testing is likely to fuel market expansion. Access to enhanced analysis, along with highly advanced and accurate testing solutions, would therefore increase product acceptance in drug screening laboratories, pushing segmental revenue.
Regional Segment Analysis of the specimen validity testing market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
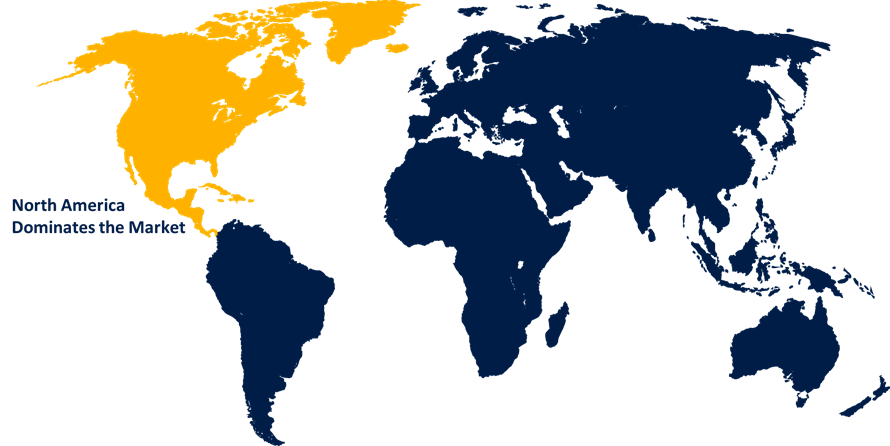
North America led the market with the largest market revenue during the forecast period
Get more details on this report -
North America is leading the significant market growth over the forecast period owing to the rapidly expanding use of illegal drugs, increasing prevalence of prescription diversion, growing population of drug addicts, and adoption of tough regulations demanding drug screening. Furthermore, greater government funding availability, increased initiatives to boost drug screening, and a strong presence of important companies are some of the reasons projected to fuel revenue development in the region.
Asia Pacific is expected to experience high revenue market growth during the forecast period due to sustained economic growth, increased level of disposable income, increasing frequency of methamphetamine and ketamine misuse, and expanding necessity for drug screening tests. Increased investment in R&D and the construction of cutting-edge healthcare facilities are also predicted to fuel revenue growth in the area.
Competitive Analysis
The report offers the appropriate analysis of the key organizations/companies involved within the global specimen validity testing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Thermo Fisher
- Sciteck
- American Bio Medica Corporation
- Alere
- Express Diagnostics
- Premier Biotech.
- Quest Diagnostics
- Alere Toxicology
- ACM Global Laboratories
- Clinical Reference Laboratory (CRL)
- SureHire
- CannAmm
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In April 2020, Nona Scientific announced the release of NonaClear 2.0, an advanced specimen validity assessment tool. This test can detect fake urine as well as numerous types of subversion. This creative product launch assisted the corporation in expanding its product portfolio and increasing its corporate income.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2022 to 2032. Spherical Insights has segmented the Global Specimen Validity Testing Market based on the below-mentioned segments:
Global Specimen Validity Testing Market, By Product and Services
- Product {Assay Kits, Reagents & Calibrators, Disposables}
- Service
Global Specimen Validity Testing Market, By Drug Class
- Laboratory Testing
- Rapid/POC Testing
Global Specimen Validity Testing Market, By End Users
- Workplace
- Drug Screening
- Pain Management
- Drug Rehabilitation Centers
Specimen Validity Testing Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Need help to buy this report?