South Korea Rapid Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Product Type (Over the Counter (OTC) Kits and Professional Kits), By Application (Blood Glucose Testing, Infectious Disease Testing, Cardiometabolic Testing, Pregnancy Fertility Testing, Fecal Occult Blood Testing, Coagulation Testing, and Toxicology Testing), and South Korea Rapid Diagnostics Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareSouth Korea Rapid Diagnostics Market Insights Forecasts to 2035
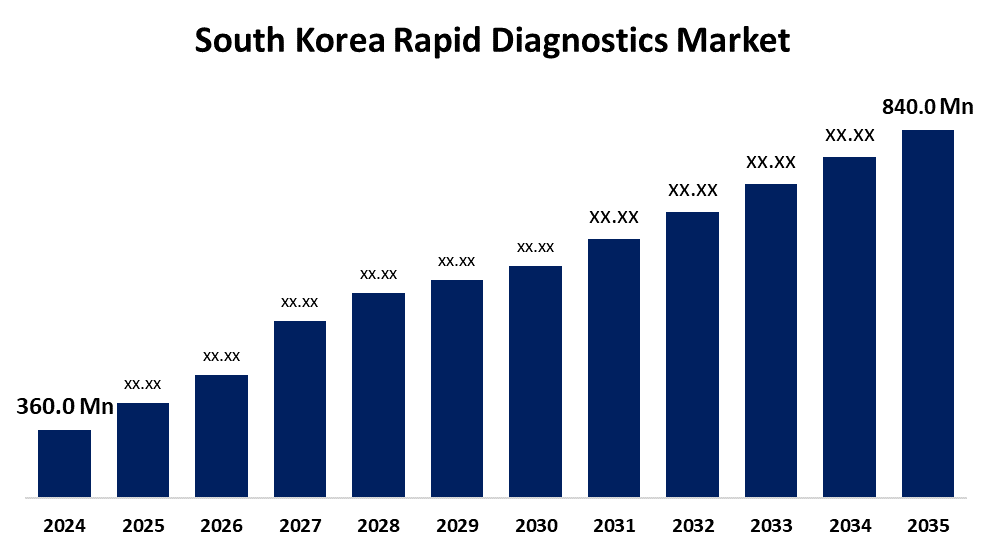
- The South Korea Rapid Diagnostics Market Size was Estimated at USD 360.0 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 8.01% from 2025 to 2035
- The South Korea Rapid Diagnostics Market Size is Expected to Reach USD 840.0 Million by 2035
Get more details on this report -
According to a Research Report Published by Spherical Insights & Consulting, the South Korea Rapid Diagnostics Market Size is Anticipated to Reach USD 840.0 Million by 2035, Growing at a CAGR of 8.01% from 2025 to 2035. Growing as a result of increased demand for point-of-care testing, particularly for chronic illnesses like diabetes and cardiovascular diseases as well as infectious diseases. Government investments in healthcare innovation, the use of AI-integrated diagnostic platforms, and growing consumer preference for early intervention and self-testing are additional factors driving growth.
Market Overview
The South Korea rapid diagnostics market refers to the domestic ecosystem for quick, point-of-care diagnostic solutions that produce results in a matter of minutes to hours is referred to as the South Korean rapid diagnostics market. Application, this market offers a lot of room for expansion, especially given the rapid advancements in technology. The steady expansion of diagnostic kits that offer precise results at the point of care and point of care testing has made investment opportunities more appealing. Additionally, the growth of self-testing and telemedicine focuses on providing patients in underserved, remote areas with quick and easy access to diagnostics. A shift towards quicker, simpler, and more reliable testing techniques is also indicated by the growing reliance on molecular diagnostics and the pertinent trends of new rapid antigen tests. Furthermore, there is a growing emphasis on public health organizations and private organizations working together to make diagnostic tests more affordable and accessible. As South America's healthcare needs are satisfied, developments in rapid diagnostics will shift to meet the region's healthcare issues and ecosystem.
Report Coverage
This research report categorizes the market for the South Korea rapid diagnostics market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the South Korea rapid diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the South Korea rapid diagnostics market.
South Korea Rapid Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 360.0 Million |
| Forecast Period: | 2024-2035 |
| Forecast Period CAGR 2024-2035 : | 8.01% |
| 2035 Value Projection: | USD 840.0 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 180 |
| Tables, Charts & Figures: | 160 |
| Segments covered: | By Product Type, By Application and COVID-19 Impact Analysis. |
| Companies covered:: | Seegene Inc., Coreline Soft, IBEX Medical Systems, 1drop Inc., Lameditech, and Others. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The South Korean Rapid Diagnostics Market Industry is being driven by the rapid development of diagnostic technologies, such as the introduction of novel assays and laboratory-on-a-chip devices. Advances in microfluidics and biosensing technologies are anticipated to improve the precision and effectiveness of quick tests with the help of regional research institutes like POSTECH and KAIST. The market is expected to grow as a result of significant improvements in the sensitivity and specificity of diagnostic tests brought about by various government-funded research grants. In order to increase the adoption of advanced rapid diagnostics in a variety of healthcare settings, the Korean government is aggressively supporting R initiatives that should increase the accessibility of these technologies to healthcare providers.
Restraining Factors
The market for rapid diagnostics in South Korea is constrained by the high cost of sophisticated kits, restricted reimbursement, and regulatory barriers. Furthermore, despite the rising demand for infectious disease screening and point-of-care testing, widespread adoption is hampered by unequal access in rural areas, data privacy issues, and a shortage of qualified staff.
Market Segmentation
The South Korea rapid diagnostics market share is classified into product type and application.
- The professional kits segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea rapid diagnostics market is segmented by product type into over the counter (OTC) and professional kits. Among these, the professional kits segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. Professional kits, which serve medical professionals who need precise testing methods for efficient patient management, continue to be essential to diagnostic accuracy and dependability. For these kits aid in early disease detection and ultimately improve health outcomes, their use in clinical settings is crucial.
- The infectious disease testing segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea rapid diagnostics market is segmented by application into blood glucose testing, infectious disease testing, cardiometabolic testing, pregnancy fertility testing, fecal occult blood testing, coagulation testing, and toxicology testing. Among these, the infectious disease testing segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. Since infectious disease testing provides quick diagnostics that can enhance patient outcomes and stop the spread of disease, it has become more popular, especially in response to pandemics. This highlights the significance of infectious disease testing in both hospital and point-of-care settings.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the South Korea rapid diagnostics market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Seegene Inc.
- Coreline Soft
- IBEX Medical Systems
- 1drop Inc.
- Lameditech
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at South Korea, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the South Korea Rapid Diagnostics Market based on the below-mentioned segments:
South Korea Rapid Diagnostics Market, By Product Type
- Over the Counter (OTC) Kits
- Professional Kits
South Korea Rapid Diagnostics Market, By Application
- Blood Glucose Testing
- Infectious Disease Testing
- Cardiometabolic Testing
- Pregnancy Fertility Testing
- Fecal Occult Blood Testing
- Coagulation Testing
- Toxicology Testing
Need help to buy this report?