South Korea Pharmaceutical Serialization Services Market Size, Share, and COVID-19 Impact Analysis, By Packaging (Primary Packaging, Secondary Packaging, and Tertiary Packaging), By Product (Hardware and Software), By Technology (Bardcodes, Radiofrequency Identification (RFID) Tags, Real-time Locating System, and Others), and South Korea Pharmaceutical Serialization Services Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareSouth Korea Pharmaceutical Serialization Services Market Insights Forecasts to 2035
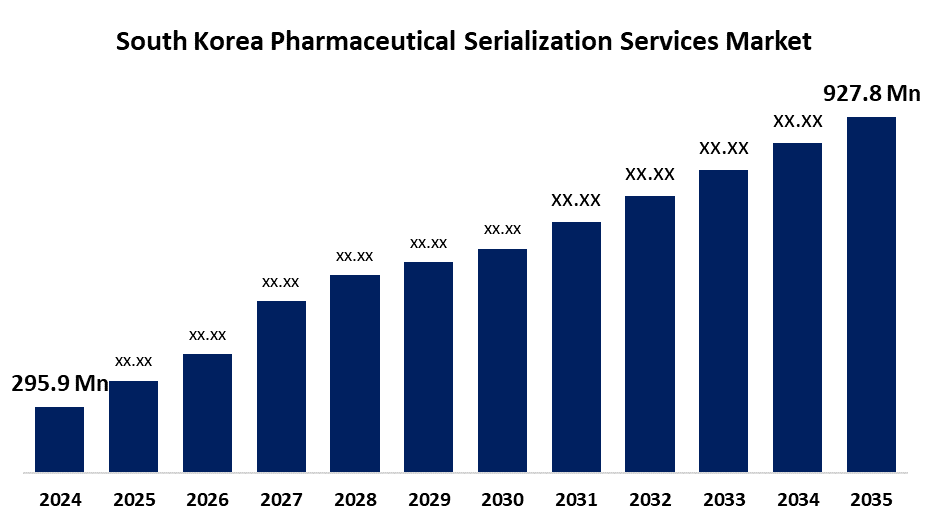
- The South Korea Pharmaceutical Serialization Services Market Size Was Estimated at USD 295.9 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 10.95% from 2025 to 2035
- The South Korea Pharmaceutical Serialization Services Market Size is Expected to Reach USD 927.8 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the South Korea Pharmaceutical Serialization Services Market is anticipated to reach USD 927.8 Million by 2035, growing at a CAGR of 10.95% from 2025 to 2035. Growing regulatory requirements, such as those requiring drug traceability and verification across the supply chain, are the main factors propelling the market's expansion. Systems for serialization are essential in the fight against fake medications because they guarantee that only authentic goods are delivered to customers.
Market Overview
To facilitate tracking and tracing across the supply chain, pharmaceutical serialization services entail giving each saleable unit of a drug product a unique, distinct code (similar to serial numbers). This procedure supports regulatory compliance, improves supply chain security, and helps guarantee product authenticity. For the implementation and operation of serialization systems, including data management, compliance assistance, and hardware and software integration, pharmaceutical businesses are looking for professional service providers. Businesses that provide blockchain-based serialization solutions are in a good position to benefit from the rising need for improved supply chain transparency and security. Regulations that specify the precise specifications for serialization, such as the kind of data to be encoded, the coding format, and the implementation schedules, are being established and enforced by governments. In the battle against fake medications, serialization and track-and-trace systems are essential because they allow for the identification of fake goods and the confirmation of product authenticity.
Report Coverage
This research report categorizes the market for South Korea pharmaceutical serialization services market based on various segments and regions forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the South Korea pharmaceutical serialization services market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment South Korea pharmaceutical serialization services market.
South Korea Pharmaceutical Serialization Services Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 295.9 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 10.95% |
| 2035 Value Projection: | USD 927.8 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 220 |
| Tables, Charts & Figures: | 123 |
| Segments covered: | By Packaging, By Product, By Technology and COVID-19 Impact Analysis |
| Companies covered:: | Samsung Biologics, Lotte Biologics, Vetter Pharma International, Recipharm, Servier CDMO, Chong Kun Dang, Daewoong Pharmaceutical, GeneMatrix, Seegene, Hanmi Pharmaceutical, SK Biopharmaceuticals, GC Pharma, Others. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis. |
Get more details on this report -
Driving Factors
The increasing prevalence of counterfeit drugs, estimated to be responsible for numerous deaths annually, underscores the need for robust serialization solutions to safeguard public health. Technological advancements, including the integration of artificial intelligence, blockchain, and Internet of Things (IoT) devices, are enhancing the capabilities of serialization systems, enabling real-time tracking, data analytics, and improved supply chain visibility. These factors collectively contribute to the market's expansion, as pharmaceutical companies seek to comply with regulations, enhance security, and improve operational efficiency.
Restraining Factors
High implementation costs, including investments in specialized equipment, software, and staff training, can be prohibitive, especially for small and medium-sized enterprises (SMEs). Integration with existing legacy systems often leads to compatibility issues, causing delays and increased expenses. Additionally, the complexity of global supply chains, varying regulatory requirements across countries, and concerns over data privacy and cybersecurity further complicate the adoption and effective implementation of serialization solutions.
Market Segmentation
The South Korea pharmaceutical serialization services market share is classified into packaging, product, and technology.
- The primary packaging segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea pharmaceutical serialization services market is segmented by packaging into primary packaging, secondary packaging, and tertiary packaging. Among these, the primary packaging segment held a significant share in 2024 and is expected to grow at a substantial CAGR during the forecast period. Primary packaging’s vital role in guaranteeing the safety and authenticity of items is primarily responsible for its expansion. The serialization of bottles, blister packs, and other immediate packaging forms the initial point of contact between the drug and the consumer is covered in this section.
- The hardware segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea pharmaceutical serialization services market is segmented by product into hardware and software. Among these, the hardware segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. Each unit is appropriately marked with a unique identity, hardware elements like printers, scanners, and labeling machines are crucial for the implementation of serialization systems. Furthermore, the adoption of cutting-edge hardware solutions is being driven by the growing need to comply with strict regulatory requirements as well as the growing desire for real-time drug tracking and verification.
- The bardcodes segment dominated share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea pharmaceutical serialization services market is segmented by technology into bardcodes, radiofrequency identification (RFID) tags, real-time locating system, and others. Among these, the bardcodes segment dominated share in 2024 and is expected to grow at a significant CAGR during the forecast period. The extensive use in tracking products, affordability, and ease of use are the reasons for their expansion. Drugs can be efficiently and accurately identified and authenticated at several points in the supply chain thanks to barcodes, especially 2D barcodes.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the South Korea pharmaceutical serialization services market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the companies' current news and developments, including product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Samsung Biologics
- Lotte Biologics
- Vetter Pharma International
- Recipharm
- Servier CDMO
- Chong Kun Dang
- Daewoong Pharmaceutical
- GeneMatrix
- Seegene
- Hanmi Pharmaceutical
- SK Biopharmaceuticals
- GC Pharma
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at South Korea, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the South Korea pharmaceutical serialization services market based on the below-mentioned segments:
South Korea Pharmaceutical Serialization Services Market, By Packaging
- Primary Packaging
- Secondary Packaging
- Tertiary Packaging
South Korea Pharmaceutical Serialization Services Market, By Product
- Hardware
- Software
South Korea Pharmaceutical Serialization Services Market, By Technology
- Bardcodes
- Radiofrequency Identification (RFID) Tags
- Real-time Locating System
- Others
Need help to buy this report?