South Korea Dengue Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Product (ELISA-based Tests, Dengue IgG/IgM Detection Kits, RT-PCR Tests, and Rapid Diagnostic Tests), By Service Type (Centralized Service and POC Service), By End-use (Home Healthcare and Clinical Labs), and South Korea Dengue Diagnostics Market Industry Trend, Forecasts to 2035
Industry: HealthcareSouth Korea Dengue Diagnostics Market Insights Forecasts to 2035
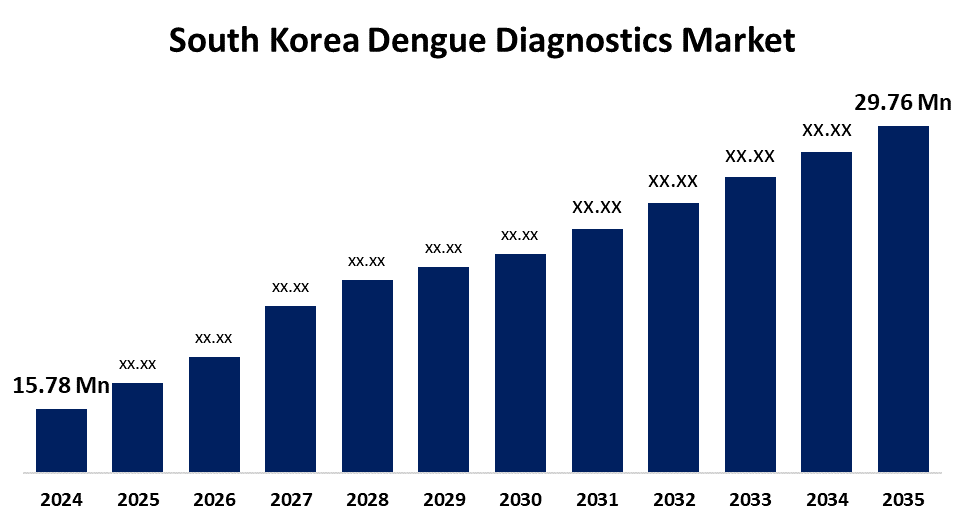
- The South Korea Dengue Diagnostics Market Size Was Estimated at USD 15.78 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 5.94% from 2025 to 2035
- The South Korea Dengue Diagnostics Market Size is Expected to Reach USD 29.76 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, The South Korea Dengue Diagnostics Market Size is anticipated to reach USD 29.76 Million by 2035, growing at a CAGR of 5.94% from 2025 to 2035. The South Korea dengue diagnostics market is experiencing escalating dengue outbreaks, technological advancements, and increased public health initiatives.
Market Overview
The dengue diagnostics market refers to the industry focused on developing and providing tools and technologies for the accurate detection and diagnosis of dengue fever. These include ELISA tests, rapid diagnostic tests, RT-PCR, and antibody detection kits. The key benefits of effective dengue diagnostics are early detection, timely treatment, reduced transmission, and improved patient outcomes. The technological advancements, rising dengue incidence, and growing demand for point-of-care testing. In South Korea, government initiatives such as public awareness campaigns, investment in healthcare infrastructure, and support for infectious disease surveillance have strengthened the diagnostics landscape. Efforts to improve early diagnosis and outbreak control, alongside partnerships with private diagnostics firms, further create favorable conditions for growth and innovation in the dengue diagnostics market.
Report Coverage
This research report categorizes the market for South Korea dengue diagnostics market based on various segments and regions forecasts revenue growth, and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the South Korea dengue diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment South Korea dengue diagnostics market.
South Korea Dengue Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 15.78 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 5.94% |
| 2035 Value Projection: | USD 29.76 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 189 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Product, By Service Type and By End-use |
| Companies covered:: | Seegene, Inc, SD Biosensor, Asan Pharm / Asan Easy Test, Boditech Med (Ichroma Dengue), InBios International (distributed in KR), Euroimmun (distributed in KR), Novatec Immundiagnostica, Roche Diagnostics, Abbott Laboratories (Panbio), RapiGEN Inc., M monitor Inc., GC Medical Science Corp., and Others |
| Pitfalls & Challenges: | Covid-19 Impact, Challenge, Future,Growth and Analysis |
Get more details on this report -
Driving Factors
The rising incidence of dengue fever due to climate change and urbanization, which increases mosquito breeding. Growing awareness about early diagnosis and improved health outcomes fuels demand for reliable testing methods. Technological advancements in diagnostic tools, such as rapid tests and molecular diagnostics, also support market growth.
Restraining Factors
The high cost of advanced diagnostic tests such as RT-PCR and molecular assays limits accessibility, especially in low-resource or rural regions. Limited healthcare infrastructure and trained personnel, particularly in underserved areas, hinder widespread adoption and accurate implementation of tests. Diagnostic inaccuracies, such as cross-reactivity in serological tests can result in false positives/negatives, undermining reliability.
Market Segmentation
The South Korea dengue diagnostics market share is classified into product, service type, and end-use.
- The ELISA-based tests segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea dengue diagnostics market is segmented by product into ELISA-based tests, dengue IgG/IgM detection kits, RT-PCR tests, and rapid diagnostic tests. Among these, the ELISA-based tests segment held a significant share in 2024 and is expected to grow at a substantial CAGR during the forecast period. The high sensitivity, specificity, and ability to detect dengue antibodies early in infection. These tests are widely used in hospitals and laboratories for accurate diagnosis and large-scale screening. Their cost-effectiveness, reliability, and capability to process multiple samples simultaneously make them a preferred choice.
- The centralized service segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea dengue diagnostics market is segmented by service type into centralized service and POC service. Among these, the centralized service segment dominated a share in 2024 and is expected to grow at a significant CAGR during the forecast period. The availability of advanced laboratory infrastructure and highly trained professionals in centralized facilities. These services offer greater accuracy, standardized testing procedures, and the capacity to handle large volumes of samples efficiently. Centralized labs are preferred for confirmatory testing using methods like ELISA and RT-PCR. As healthcare systems emphasize quality and reliability in diagnostics.
- The home healthcare segment dominated a share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea dengue diagnostics market is segmented by end-use into home healthcare, and clinical labs. Among these, the home healthcare segment dominated a share in 2024 and is expected to grow at a significant CAGR during the forecast period. The increasing consumer preference for at-home testing, driven by convenience, faster results, and reduced risk of exposure to infections in clinical settings. The widespread availability of rapid diagnostic tests has made self-testing more accessible and reliable. Additionally, technological advancements and growing awareness of early dengue detection have fueled demand.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the South Korea dengue diagnostics market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the companies' current news and developments, including product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Seegene, Inc
- SD Biosensor
- Asan Pharm / Asan Easy Test
- Boditech Med (Ichroma Dengue)
- InBios International (distributed in KR)
- Euroimmun (distributed in KR)
- Novatec Immundiagnostica
- Roche Diagnostics
- Abbott Laboratories (Panbio)
- RapiGEN Inc.
- M monitor Inc.
- GC Medical Science Corp.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at South Korea, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the South Korea dengue diagnostics market based on the below-mentioned segments:
South Korea Dengue Diagnostics Market, By Product
- ELISA-based Tests
- Dengue IgG/IgM Detection Kits
- RT-PCR Tests
- Rapid Diagnostic Tests
South Korea Dengue Diagnostics Market, By Service Type
- Centralized Service
- POC Service
South Korea Dengue Diagnostics Market, By End-use
- Home Healthcare
- Clinical Labs
Need help to buy this report?