South Korea Companion Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Product and Services (Assays, Kits, & Reagents, Instruments & Systems, and Software & Services), By Technology (Polymerase Chain Reaction (PCR) and Next-Generation Sequencing (NGS)), and South Korea Companion Diagnostics Market Industry Trend, Forecasts to 2035
Industry: Information & TechnologySouth Korea Companion Diagnostics Market Size Insights Forecasts to 2035
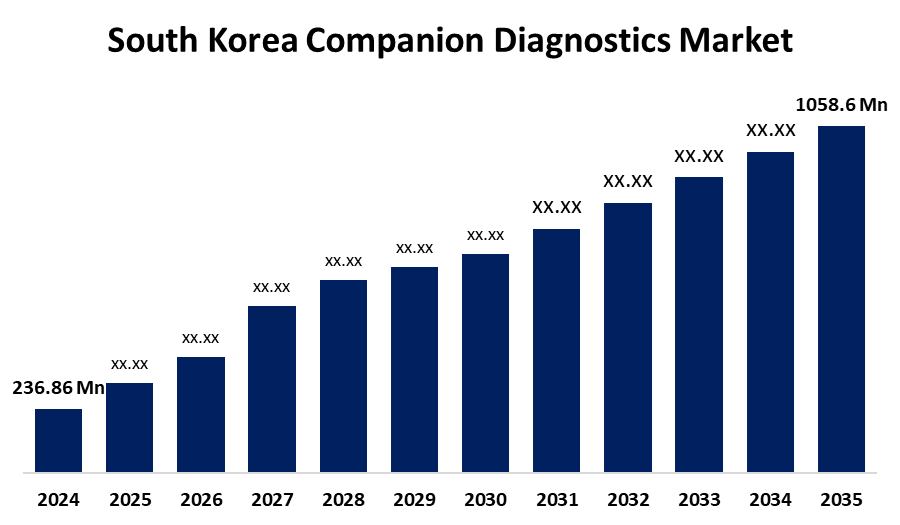
- The South Korea Companion Diagnostics Market Size Was Estimated at USD 236.86 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 14.58% from 2025 to 2035
- The South Korea Companion Diagnostics Market Size is Expected to Reach USD 1058.6 Million by 2035
Get more details on this report -
According to a Research Report Published by Spherical Insights & Consulting, the South Korea Companion Diagnostics Market Size is anticipated to reach USD 1058.6 million by 2035, growing at a CAGR of 14.58% from 2025 to 2035. The identification of biomarkers that forecast responses to particular medications, companion diagnostics play a critical role in customizing treatments for each patient.
Market Overview
The companion diagnostics market refers to medical tests designed to identify patients most likely to benefit from a specific drug or therapy, ensuring personalized and targeted treatment. These diagnostics play a crucial role in precision medicine by improving treatment outcomes, minimizing adverse effects, and reducing healthcare costs. The enhanced drug efficacy, better patient stratification, and faster clinical decision-making. The market is expanding with the rise of targeted therapies, cancer treatments, and next-generation sequencing technologies. In South Korea, government initiatives supporting biotech innovation, personalized medicine, and genomic research are driving market growth. Programs that promote public-private partnerships, regulatory streamlining, and investment in precision healthcare are positioning the country as a regional leader in the adoption of companion diagnostics.
Report Coverage
This research report categorizes the market for South Korea companion diagnostics market based on various segments and regions forecasts revenue growth, and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the South Korea companion diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment South Korea companion diagnostics market.
South Korea Companion Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 236.86 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 14.58% |
| 2035 Value Projection: | USD 1058.6 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 136 |
| Tables, Charts & Figures: | 115 |
| Segments covered: | By Product and Services, By Technology and COVID-19 Impact Analysis |
| Companies covered:: | T&L Co., Ltd., DUK-IN, SeohanCare Co., Ltd., Solco Biomedical Co., Ltd., Taeyeon Medical, TDM Co., Ltd., U&I Corporation, INFINITT Healthcare, Cybermed Inc., OSTEOSYS Co., Ltd., CG Bio Inc., Woosam Medical, and Other key vendors |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The growing demand for personalized medicine and targeted therapies, particularly in oncology. As targeted treatments become more prevalent, accurate diagnostic tools like PCR and NGS enable precise patient stratification, improving therapeutic efficacy and minimizing adverse reactions. Advances in genomic research and biomarker discovery further expand testing capabilities. Additionally, increasing healthcare investments, supportive regulatory frameworks, and rising awareness among healthcare providers and patients contribute to market growth as precision diagnostics become integral to modern care.
Restraining Factors
High development costs, including clinical validation, regulatory submissions, and co-development with therapies, limit new entrants, especially among smaller firms. Stringent and complex regulatory requirements often coordinated with drug approvals can delay market entry and add burden. Inconsistent reimbursement policies limit test accessibility, as payers vary widely in coverage decisions.
Market Segmentation
The South Korea companion diagnostics market share is classified into product and services and technology.
- The assays segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea companion diagnostics market is segmented by product and services into assays, kits, & reagents, instruments & systems, and software & services. Among these, the assays segment held a significant share in 2024 and is expected to grow at a substantial CAGR during the forecast period. Assays, kits & reagents, equipment & systems, and software & services are the product and service categories into which the South Korean companion diagnostics market is divided. The assays segment, which accounted for a sizeable portion of these in 2024, is anticipated to expand at a strong compound annual growth rate (CAGR) during the course of the forecast period.
- The polymerase chain reaction (PCR) segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea companion diagnostics market is segmented by technology into polymerase chain reaction (PCR) and next-generation sequencing (NGS). Among these, the polymerase chain reaction (PCR) dominated a share in 2024 and is expected to grow at a significant CAGR during the forecast period. Its high sensitivity, accuracy, and cost-effectiveness in detecting genetic mutations. PCR is widely used in oncology for identifying biomarkers that guide targeted therapies, making it essential in personalized medicine. Its established clinical utility, faster turnaround times, and compatibility with existing lab infrastructure further support its dominance.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the South Korea companion diagnostics market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborate analysis focusing on the companies' current news and developments, including product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- T&L Co., Ltd.
- DUK-IN
- SeohanCare Co., Ltd.
- Solco Biomedical Co., Ltd.
- Taeyeon Medical
- TDM Co., Ltd.
- U&I Corporation
- INFINITT Healthcare
- Cybermed Inc.
- OSTEOSYS Co., Ltd.
- CG Bio Inc.
- Woosam Medical
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at South Korea, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the South Korea companion diagnostics market based on the below-mentioned segments:
South Korea Companion Diagnostics Market, By Product and Services
- Assays
- Kits, & Reagents
- Instruments & Systems
- Software & Services
South Korea Companion Diagnostics Market, By Technology
- Polymerase Chain Reaction (PCR)
- Next-Generation Sequencing (NGS)
Need help to buy this report?