South Korea Cardiovascular Devices Market Size, Share, and COVID-19 Impact Analysis, By Type (Cardiac Rhythm Management Devices, Interventional Cardiac Devices, Automated External Defibrillators (AED), Cardiac Ablation Catheters, Cardiac Pacemakers, Cardiac Angioplasty Devices, Implantable Cardioverter Defibrillators (ICD), Prosthetics (Artificial) Heart Valves, Stents, Ventricular Assist Devices), By Technology (Cardiopulmonary Bypass Products, Minimally Invasive Cardiac Surgery, Valve Prosthesis and Repair, Cardiac Assist Devices), and South Korea Cardiovascular Devices Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareSouth Korea Cardiovascular Devices Market Insights Forecasts to 2035
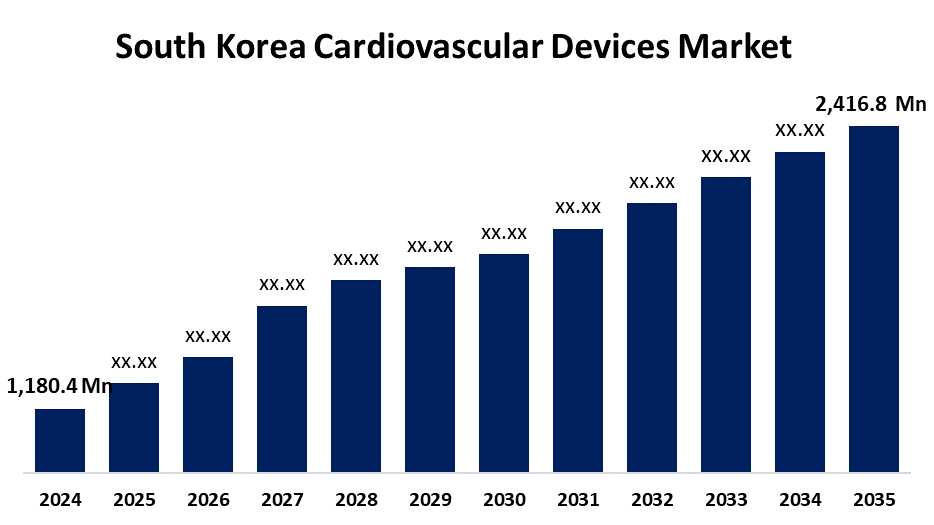
- The South Korea Cardiovascular Devices Market Size was estimated at USD 1,180.4 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 6.73% from 2025 to 2035
- The South Korea Cardiovascular Devices Market Size is Expected to Reach USD 2,416.8 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the South Korea Cardiovascular Devices Market is anticipated to reach USD 2,416.8 million by 2035, growing at a CAGR of 6.73% from 2025 to 2035. A few of the major factors propelling the market expansion in South Korea are the rising prevalence of cardiovascular disease, the expanding use of telemedicine services for remote monitoring and management of cardiovascular conditions, and growing awareness of the significance of maintaining good heart health.
Market Overview
The South Korea cardiovascular devices market encompasses a wide variety of medical devices intended to identify, track, and treat heart and blood vessel disorders are included in the South Korean cardiovascular devices market. This market covers both therapeutic and diagnostic devices used nationwide in ambulatory surgical centers, specialty clinics, and hospitals. An aging population, the rising incidence of cardiovascular diseases, and improvements in medical technology are the main factors driving the demand for these devices. Additionally, the market is anticipated to grow as a result of the following factors: the use of remote monitoring devices, the integration of AI and ML, the adoption of minimally invasive procedures, improvements in imaging technologies, etc. Additionally, developments in genomics and biomarker research are opening the door to precision and personalized medicine in cardiovascular care. In order to improve efficacy and results, treatments and interventions are increasingly being customized to the unique genetic profile and condition of each patient. Moreover, the market for cardiovascular devices in South Korea has a lot of potential due to the country's rapidly aging population. Because cardiovascular diseases are more common in older adults, there is a greater need for diagnostic and therapeutic devices, which supports the revenue of the cardiovascular devices market in South Korea.
Report Coverage
This research report categorizes the market for the South Korea cardiovascular devices market based on various segments and regions and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the South Korea cardiovascular devices market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the South Korea cardiovascular devices market.
South Korea Cardiovascular Devices Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 1,180.4 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 6.73% |
| 2035 Value Projection: | USD 2,416.8 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 215 |
| Tables, Charts & Figures: | 126 |
| Segments covered: | By Type, By Technology and COVID-19 Impact Analysis |
| Companies covered:: | Medtronic PLC, Boston Scientific Corporation, Abbott Laboratories, GE Healthcare, Siemens Healthineers AG, Others. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis. |
Get more details on this report -
Driving Factors
The developing medical business, the growing demand for dependable and affordable cardiovascular equipment, the growing preference for improved treatment alternatives, and the growing use of telemedicine are some of the major drivers driving the industry's expansion. Additionally, ongoing developments in cardiovascular device technologies such as less invasive surgical methods, improved imaging methods, and safer and more effective stent designs also support the expansion of the South Korean cardiovascular devices industry.
Restraining Factors
Regulatory barriers, technological adaptation, healthcare infrastructure constraints, and intense competition are some of the issues the market is dealing with.
Market Segmentation
The South Korea cardiovascular devices market share is classified into type and technology.
- The rhythm management devices segment segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea cardiovascular devices market is segmented by type into cardiac rhythm management devices, interventional cardiac devices, automated external defibrillators (AED), cardiac ablation catheters, cardiac pacemakers, cardiac angioplasty devices, implantable cardioverter defibrillators (ICD), prosthetics (artificial) heart valves, stents, and ventricular assist devices. Among these, the cardiac rhythm management devices segment segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. Heart rhythm abnormalities like bradycardia (slow heart rate) and tachycardia (fast heart rate) are treated with a cardiac rhythm management device. On the other hand, minimally invasive procedures that treat coronary artery disease and other cardiovascular conditions use interventional cardiac devices. Additionally, in cases of sudden cardiac arrest, an electric shock is administered via an AED to restore normal cardiac rhythm.
- The cardiopulmonary bypass products segment segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The South Korea cardiovascular devices market is segmented by technology into cardiopulmonary bypass products, minimally invasive cardiac surgery, valve prosthesis and repair, and cardiac assist devices. Among these, the cardiopulmonary bypass products segment segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is driven by the need for open heart surgeries to treat conditions like congenital heart defects, valve problems, and coronary artery disease, as well as the prevalence of cardiovascular diseases. However, patient demand for less invasive procedures, quicker recovery periods, and improvements in surgical technology all influence the use of minimally invasive techniques in heart surgery. Furthermore, the aging population and the prevalence of valvular heart diseases have an impact on the South Korean market for valve prosthesis and repair devices. Additionally, the rising incidence of heart failure and the need for cutting-edge treatment options for patients with end-stage heart disease are driving the nation's demand for cardiac assist devices.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the South Korea cardiovascular devices market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Medtronic PLC
- Boston Scientific Corporation
- Abbott Laboratories
- GE Healthcare
- Siemens Healthineers AG
- Others
Recent Developments:
- In April 2024, V.V.T. Med Ltd., a medical devices company, completed the GMP certification and registration of Novel-Thermal, an anesthesia-free treatment for varicose veins, and received approval from the Korean Ministry of Food and Drug Safety.
- In February 2023, Heart Test Laboratories, an AI-powered medical technology firm, received a patent application from South Korea for its advanced ECG/EKG technology, aimed at earlier identification of heart illness to improve patient outcomes.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at South Korea, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the South Korea Cardiovascular Devices Market based on the below-mentioned segments:
South Korea Cardiovascular Devices Market, By Type
- Cardiac Rhythm Management Devices
- Interventional Cardiac Devices
- Automated External Defibrillators (AED)
- Cardiac Ablation Catheters
- Cardiac Pacemakers
- Cardiac Angioplasty Devices
- Implantable Cardioverter Defibrillators (ICD)
- Prosthetics (Artificial) Heart Valves
- Stents
- Ventricular Assist Devices
South Korea Cardiovascular Devices Market, By Technology
- Cardiopulmonary Bypass Products
- Minimally Invasive Cardiac Surgery
- Valve Prosthesis and Repair
- Cardiac Assist Devices
Need help to buy this report?