Global Pyrogen Testing Market Size, Share, and COVID-19 Impact Analysis, By Product & Service (Reagents & Kits, Instruments, Services), By Test Type (LAL Test, Rabbit Pyrogen Test, Others), By End User (Food & Beverage Companies, Medical Devices Companies, Pharmaceuticals & Biotechnology Companies, Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 – 2033
Industry: HealthcareGlobal Pyrogen Testing Market Insights Forecasts to 2033
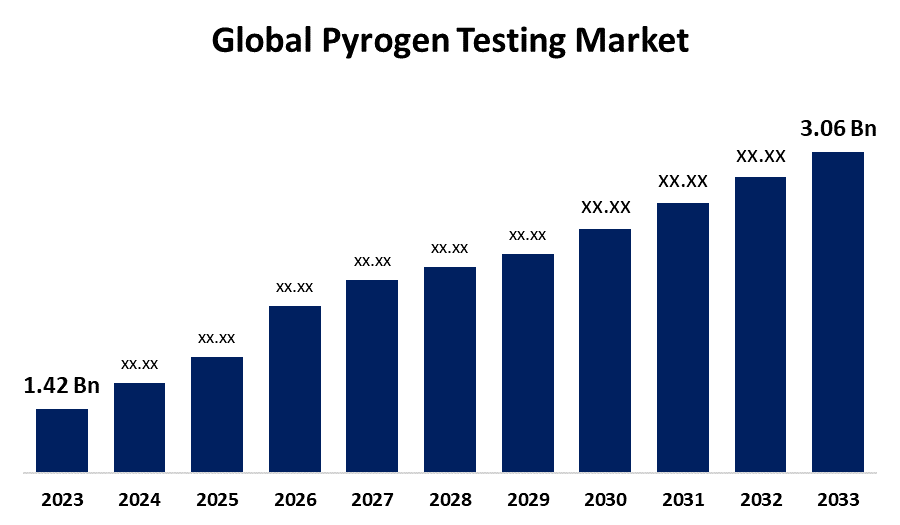
- The Global Pyrogen Testing Market Size was Valued at USD 1.42 Billion in 2023
- The Market Size is Growing at a CAGR of 7.98% from 2023 to 2033
- The Worldwide Pyrogen Testing Market Size is Expected to Reach USD 3.06 Billion by 2033
- Asia Pacific Market is expected to grow the fastest during the forecast period
Get more details on this report -
The Global Pyrogen Testing Market Size is anticipated to exceed USD 3.06 Billion by 2033, Growing at a CAGR of 7.98% from 2023 to 2033.
Market Overview
Pyrogens are fever-producing substances or agents, which are metabolic products of microorganisms. Pyrogen can be classified into two categories: endogenous pyrogen & exogenous pyrogen. Endogenous pyrogen is released due to phagocytosis, wherein bacteria are ingested by phagocytes, a special cell, which protects the body from harmful agents. The secreted endogenous pyrogen stimulates the hypothalamus in the brain, raising the body's temperature. They come from different sources, including microorganisms like bacteria, viruses, and fungi, as well as from the environment, manufacturing processes, or certain products. Pyrogen testing is an important process in pharmaceutical and medical device manufacturing to detect and measure the presence of pyrogen in products. The Limulus Amebocyte Lysate (LAL) test, is commonly used for pyrogen testing to identify and measure the levels of pyrogens in products. The market for pyrogen testing is growing at a steady pace due to rising demand for pharmaceutical products worldwide. Chronic diseases like cancer and diabetes increase drug use, necessitating pyrogen testing as the need for safety increases. In addition, the strict regulatory guidelines established by government agencies such as the U.S. The Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) have all mandated the use of pyrogen testing as part of the pharmaceutical sector's drug development and manufacturing processes, which has boosted the growth of pyrogen testing market during the forecast period.
Report Coverage
This research report categorizes the market for the global pyrogen testing market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global pyrogen testing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global pyrogen testing market.
Global Pyrogen Testing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 1.42 Billion |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 7.98% |
| 2033 Value Projection: | USD 3.06 Billion |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Product & Service, By Test Type, By End User, By Region |
| Companies covered:: | Wuxi AppTec, GenScript Biotech Corporation, Lonza Group AG, Associates of Cape Cod, Inc., Charles River Laboratories, Almac Group, SEIKAGAKU Corporation, bioMérieux SA, Thermo Fisher Scientific Inc., Pacific Biolabs, Fujifilm Holdings Corporation, STERIS plc, Indoor Biotechnologies, Inc., Eurofins Scientific SE, Merck KGaA, and Other Key Vendors. |
| Pitfalls & Challenges: | COVID-19 Empact,Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Last two decades increasing the prevalence of major infectious diseases and pandemics led to an increase in the demand for the pyrogen testing market. Rapidly growing pharmaceutical and biotechnology industries and the rising number of new therapeutics launches are anticipated to boost demand for the global pyrogen testing market. The investment in R&D through the government and manufacturing companies for the development of novel drugs and therapies across the world is increasing, owing to the rising prevalence of chronic and infectious diseases such as diabetes, cancer, heart disease, and TB. Hence, the rising demand for novel medicine and therapeutics is driving the global pyrogen testing market growth. With significant advances in pharmaceutical, and biotechnology sectors and rising life sciences R&D spending, the global pyrogen testing market is growing rapidly. In addition, patients with cancer and immunologic disorders are increasingly turning to individualized treatment based on clinical and genetic factors. As a result, one of the main factors driving demand for the pyrogen testing market is an increase in personalized medicine during the forecast period.
Restraining Factors
One of the major restraining factors is the strict government rules and regulations. The high costs associated with pyrogen testing, it is a necessary step in ensuring the safety and efficacy of healthcare products. Limited awareness of pyrogen testing in developing nations refers to the lack of knowledge and awareness of the necessity of this technique in providing the safety of pharmaceutical products and medical devices. Strict government regulations affecting the manufacture, distribution, marketing, and sale of medical devices and medications are projected to hinder growth in the global pyrogen testing market during the forecast period.
Market Segmentation
The global pyrogen testing market share is classified into product & service, test type, and end-user.
- The reagents & kits segment is expected to hold the largest share of the global pyrogen testing market during the forecast period.
Based on the product & service, the global pyrogen testing market is divided into reagents & kits, instruments, and services. Among these, the reagents & kits segment is expected to hold the largest share of the global pyrogen testing market during the forecast period. The segmental growth can be attributed to the widespread availability and user-friendly nature, providing researchers, laboratories, and pharmaceutical manufacturers with convenient tools for detecting and quantifying pyrogen. The requirement for efficiency, precision, and scalability in testing processes, as new instruments provide faster turnaround times, reduce human error, and improve overall pyrogen testing dependability.
- The LAL test segment is expected to hold the largest share of the global pyrogen testing market during the forecast period.
Based on the test type, the global pyrogen testing market is divided into LAL test, rabbit pyrogen test, and others. Among these, the LAL test segment is expected to hold the largest share of the global pyrogen testing market during the forecast period. Because of the high adoption of LAL tests, they are highly sensitive and specific making it the preferred choice for pyrogen testing in pharmaceutical and biotechnology industries. LAL test is highly accurate and reliable which is vital in ensuring the safety of pharmaceutical products and medical devices. This segment's expansion is primarily due to the increased preference for kit-based testing and frequent requirements for large numbers of assays, kits, and reagents.
- The pharmaceuticals & biotechnology company segment is expected to hold the largest share of the global pyrogen testing market during the forecast period.
Based on the end user, the global pyrogen testing market is divided into food & beverage companies, medical devices companies, pharmaceuticals & biotechnology companies, and others. Among these, the pharmaceuticals & biotechnology company segment is expected to hold the largest share of the global pyrogen testing market during the forecast period. Growth can be attributed to the increasing production of pharmaceutical, biopharmaceutical, and other biologic products. Also, the increasing focus on research and development in the pharmaceutical and biotechnology industries has resulted in a higher acceptance rate of pyrogen testing. Furthermore, the rising preference for personalized medicine and the increasing incidence of infectious and chronic diseases are driving the market growth in this category.
Regional Segment Analysis of the Global Pyrogen Testing Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- Latin America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
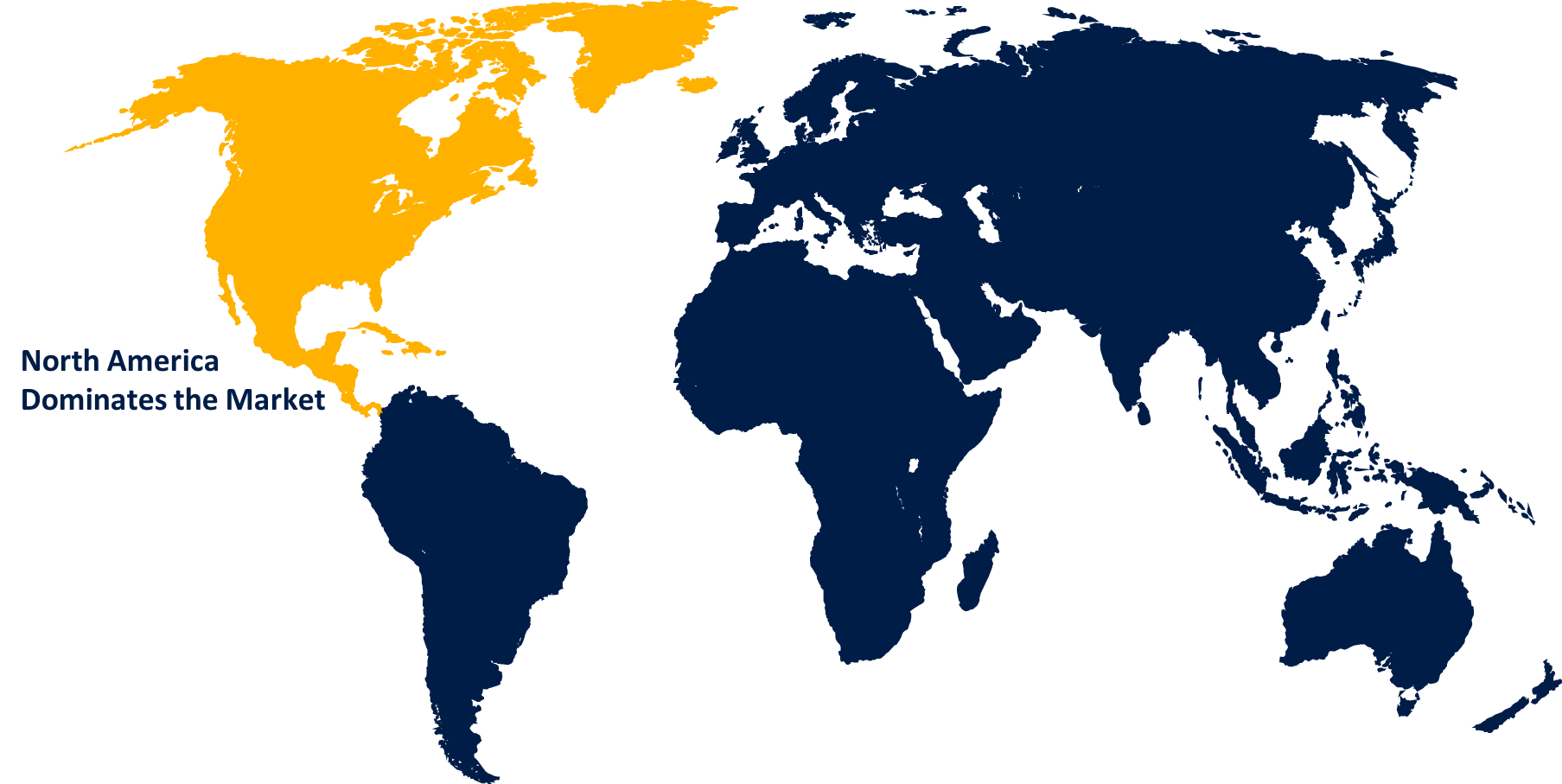
North America is anticipated to hold the largest share of the global pyrogen testing market over the predicted timeframe.
Get more details on this report -
North America is anticipated to hold the largest share of the global pyrogen testing market over the predicted timeframe. The prevalence of chronic and infectious diseases, the presence of pharmaceutical and biotechnological companies, and the advancement in research and development. Also, the presence of well-developed healthcare infrastructure, better regulatory framework pharmaceutical manufacturing process, and a large focus on new drug development to boost the pyrogen testing market. Other significant factors driving regional market expansion include expanding pharmaceutical drug pipelines, a rise in drug approvals, and increased awareness of product and food safety concerns. There is an increasing demand for safe and effective medications to treat these chronic disorders, and pyrogen testing is an important step in offering their safety and efficacy.
Asia Pacific is expected to grow at the fastest pace in the global pyrogen testing market during the forecast period. Strict government rules and regulations for drug development. Expansion of pharmaceutical and biotechnology sectors and increasing investment in healthcare infrastructure in this region.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global pyrogen testing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of overall competition within the market.
List of Key Companies
- Wuxi AppTec
- GenScript Biotech Corporation
- Lonza Group AG
- Associates of Cape Cod, Inc.
- Charles River Laboratories
- Almac Group
- SEIKAGAKU Corporation
- bioMérieux SA
- Thermo Fisher Scientific Inc.
- Pacific Biolabs
- Fujifilm Holdings Corporation
- STERIS plc
- Indoor Biotechnologies, Inc.
- Eurofins Scientific SE
- Merck KGaA
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Developments
- In October 2023, Lonza announced the launch of two monocyte activation test (MAT) systems: PyroCell MAT human serum (HS) rapid system and PyroCell MAT rapid system.
- In June 2023, Ellab A/S declared its acquisition by Novo Holdings. This acquisition will expand Novo Holdings' business in the pyrogen testing market.
- In January 2022, Eurofins introduced PFAS Exposure in the United States. It is the first direct-to-consumer at-home test to detect PFAS levels in a person's blood and identify 47 of the PFAS 'forever chemical' components.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global pyrogen testing market based on the below-mentioned segments:
Global Pyrogen Testing Market, By Product & Service
- Reagents & Kits
- Instruments
- Services
Global Pyrogen Testing Market, By Test Type
- LAL Test
- Rabbit Pyrogen Test
- Others
Global Pyrogen Testing Market, By End User
- Food & Beverage Companies
- Medical Devices Companies
- Pharmaceuticals & Biotechnology Companies
- Others
Global Pyrogen Testing Market, By Industry Vertical
- Construction & Real Estate
- Energy & Utilities
- Transportation & Logistics
- Retail
- Government & Public Sector
- Manufacturing
- Others
Global Pyrogen Testing Market, By Region
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Need help to buy this report?