Global Preterm Birth Diagnostic Test Kits Market Size, Share, and COVID-19 Impact Analysis, By Product Type (Fetal Fibronectin Test Kits (fFN}, Placental Alpha Micro-Globulin-1 Test Kits {PAMG-1}, Insulin Like Growth Factor Binding Protein-1 Test Kits {IGFBP-1}, & Others), By Sample Type (Blood Sample, Urine Sample, & Vaginal Discharge Sample), By End User (Hospitals, Diagnostic Centers, Clinics, & Others), By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2022 - 2032
Industry: HealthcareGlobal Preterm Birth Diagnostic Test Kits Market Insights Forecasts to 2032
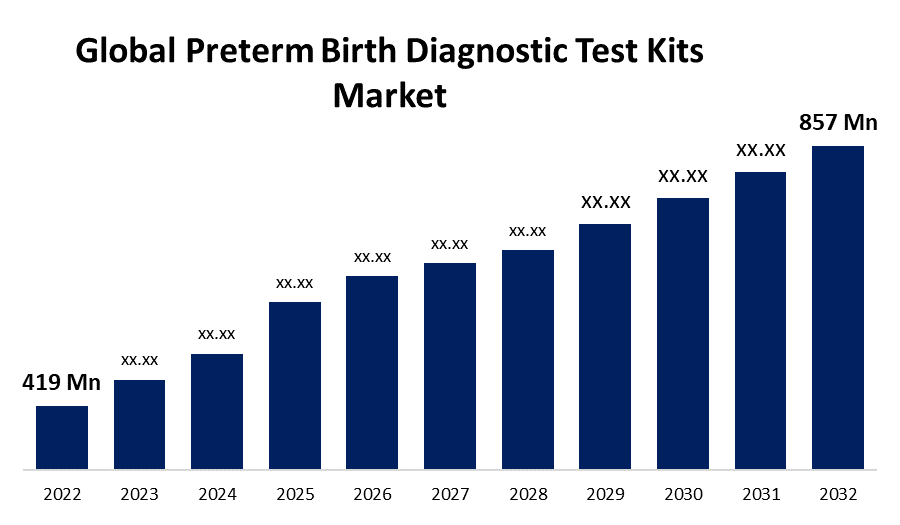
- The Global Preterm Birth Diagnostic Test Kits Market Size was valued at USD 419 Million in 2022.
- The Global Preterm Birth Diagnostic Test Kits market is growing at a CAGR of 7.4% from 2022 to 2032.
- The worldwide Preterm Birth Diagnostic Test Kits Market size is expected to reach USD 857 Million by 2032.
- Asia Pacific is expected to grow the fastest during the forecast period.
Get more details on this report -
The Global Preterm Birth Diagnostic Test Kits Market Size is expected to reach USD 857 million by 2032, at a CAGR of 7.4% during the forecast period 2022 to 2032.
Market Overview
Preterm birth (also known as neonatal birth) occurs when the baby is born before the 37th week of pregnancy. Premature newborns get particular attention during the first four weeks. Premature newborns frequently have health complications such as respiratory difficulties or weight growth. They require immediate medical treatment and a diagnosis. Preemies, or premature newborns, can be born with mental and physical problems. Many factors can contribute to preterm labor. Preterm birth is defined as a birth that happens before the 37th week of gestation. Premature birth is another word for preterm birth. It refers to a baby delivered before 37 weeks of gestation. Preemies and press are other terms for preterm newborns. A preterm birth occurs before 37 weeks of gestation. Full-term delivery occurs at 40 weeks.
Report Coverage
This research report categorizes the global preterm birth diagnostic test kits market based on various segments and regions and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global preterm birth diagnostic test kits market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global preterm birth diagnostic test kits market. Technological innovation and advancement will further optimize the performance of the product, enabling it to acquire a wider range of applications in the downstream market.
Global Preterm Birth Diagnostic Test Kits Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2022 |
| Market Size in 2022: | USD 419 Million |
| Forecast Period: | 2022-2032 |
| Forecast Period CAGR 2022-2032 : | 7.4% |
| 2032 Value Projection: | USD 857 Million |
| Historical Data for: | 2018-2021 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 130 |
| Segments covered: | By Product Type, By Sample Type, By End User, By Regionand COVID-19 Impact Analysis |
| Companies covered:: | Qiagen N.V., Hologic, Inc., Cooper Surgical Inc., Abbott, Medixbiochemica, Sera Prognostics, Clinical Innovations, LLC, Biosynex, NX Prenatal, Inc., and IQ Products And Other Key Venders |
| Pitfalls & Challenges: | COVID-19 has the potential to impact the global market |
Get more details on this report -
Driving Factors
The rising prevalence of preterm births is accelerating market expansion. Furthermore, raising awareness assists pregnant women, their families, and healthcare professionals in recognizing the signs and symptoms of premature labor. Early detection allows for immediate medical intervention, perhaps avoiding or effectively treating preterm delivery. Increased knowledge can also fuel advocacy initiatives targeted at influencing healthcare legislation, funding, and research on preterm birth prevention, treatment, and long-term outcomes. It promotes collaboration among healthcare professionals, legislators, and community groups in addressing the difficulties associated with premature births, ultimately boosting the market for preterm birth diagnostic test kits. Furthermore, numerous market participants are focusing on research and development operations to produce innovative preterm birth diagnostic test kits.
Restraining Factors
Companies that make and promote pregnancy-related diagnostic goods and services may pose strong competition for preterm birth diagnostic kits. Furthermore, clinical exams, ultrasounds, and other tests and assessments are commonly used by healthcare practitioners to diagnose pre-term birth which may restrain the market growth.
Market Segmentation
- In 2022, the fetal fibronectin test kits (fFN) segment is dominating the market with the largest market share during the forecast period.
On the basis of product type, the global preterm birth diagnostic test kits market is segmented into fetal fibronectin test kits (fFN), placental alpha micro-globulin-1 test kits {PAMG-1}, insulin like growth factor binding protein-1 test kits {IGFBP-1. Throughout these segments, the fetal fibronectin test kits (fFN) segment is dominating the market with the largest revenue share during the forecast period due to the fFN test has a high sensitivity for identifying fetal fibronectin, a protein that functions as a biomarker for preterm delivery. It can reliably identify women who are at a higher risk of giving birth prematurely. Furthermore, a rise in product availability, as well as numerous other firms attempting to develop novel technologies, will assist the segment's revenue growth in the next years.
- In 2022, the blood sample segment is influencing the largest market share over the forecast period.
Based on the sample type, the global preterm birth diagnostic test kits market is bifurcated into different categories such as blood samples, urine samples, & vaginal discharge samples. Among these segments, the blood sample segment is dominating the market during the forecast period owing to the Blood-based diagnostics have shown good diagnostic accuracy in detecting biomarkers and indications linked to premature delivery. These tests can detect particular proteins, hormones, or genetic markers in maternal blood that may indicate a higher risk of preterm delivery. As a result, the aforementioned variables will fuel segmental growth.
- In 2022, the diagnostic centers segment is dominating the market with the largest market share over the forecast period.
Based on end users, the global preterm birth diagnostic test kits market is segmented into hospitals, diagnostic centers, clinics, & others. Among these segments, the diagnostic centers segment is expected to have significant development potential over the analysis period. The surge in laboratory tests and increased demand for diagnostics laboratories due to better technology and cost-effective treatment will drive market expansion. Additionally, these centers provide early and accurate illness detection and patient care, which is expected to boost segmental growth in the future years.
Regional Segment Analysis of the Preterm Birth Diagnostic Test Kits market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
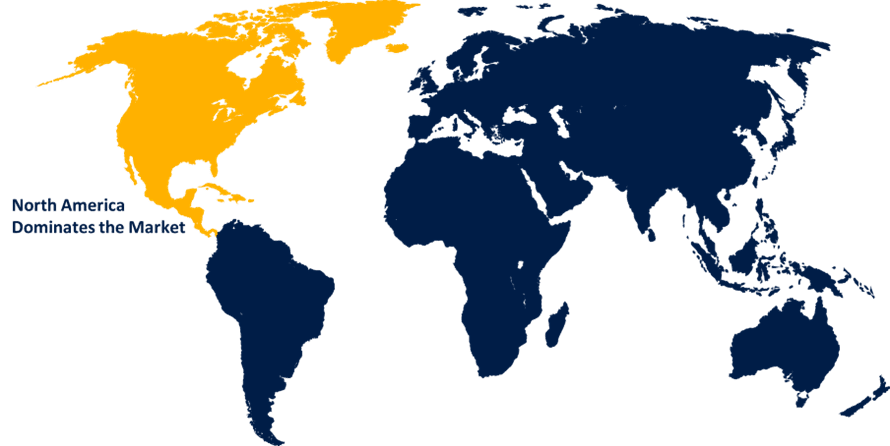
North America influenced the market with the largest market share during the forecast period
Get more details on this report -
North America is influencing significant market growth during the forecast period due to the affordability of preterm birth diagnostic test kits among the targeted customer base, as well as continuous technical improvements, which are the primary drivers of the regional preterm birth diagnostic test kits industry's growth. Furthermore, the region's high healthcare expenditure on healthcare management and growing disposable income would boost the market for preterm birth diagnostic test kits in North America. Furthermore, the large presence of well-established market competitors, as well as the favorable regulatory environment, result in several product approvals and launches, notably in the United States; as a consequence, the potential for market expansion will stimulate in the coming years.
Asia Pacific is expected to experience high revenue market growth during the forecast period due to the massive populations in India and China. Furthermore, the growing frequency of preterm births in the region's emerging countries is considerably establishing a potential market in these regions, resulting in rapid market expansion.
Competitive Analysis
The report offers the appropriate analysis of the key organizations/companies involved within the global preterm birth diagnostic test kits market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Qiagen N.V.
- Hologic, Inc.
- Cooper Surgical Inc.
- Abbott
- Medixbiochemica
- Sera Prognostics
- Clinical Innovations, LLC
- Biosynex
- NX Prenatal, Inc.
- IQ Products
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In June 2021, Medix Biochemica announced the establishment of 'MedixMDx,' a new molecular diagnostic reagents segment offering a wide variety of products and services. Such measures are expected to boost market growth.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2022 to 2032. Spherical Insights has segmented the Global Preterm Birth Diagnostic Test Kits Market based on the below-mentioned segments:
Global Preterm Birth Diagnostic Test Kits Market, Product Type
- Fetal fibronectin test kits (fFN)
- Placental alpha micro-globulin-1 test kits (PAMG-1)
- Insulin like growth factor binding protein-1 test kits (IGFBP-1)
- Others
Global Preterm Birth Diagnostic Test Kits Market, By Sample Type
- Blood sample
- Urine sample
- Vaginal discharge sample
Global Preterm Birth Diagnostic Test Kits Market, By End User
- Hospitals
- Diagnostic centers
- Clinics
- Others
Preterm Birth Diagnostic Test Kits Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- Uk
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Need help to buy this report?