Global Oncology Clinical Trials Market Size, Share, and COVID-19 Impact Analysis, By Phase Type (Phase I, Phase II, Phase III, and Phase IV), By Study Design (Interventional Studies, Observational Studies, and Expanded Access Studies), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2025 - 2035
Industry: HealthcareGlobal Oncology Clinical Trials Market Insights Forecasts to 2035
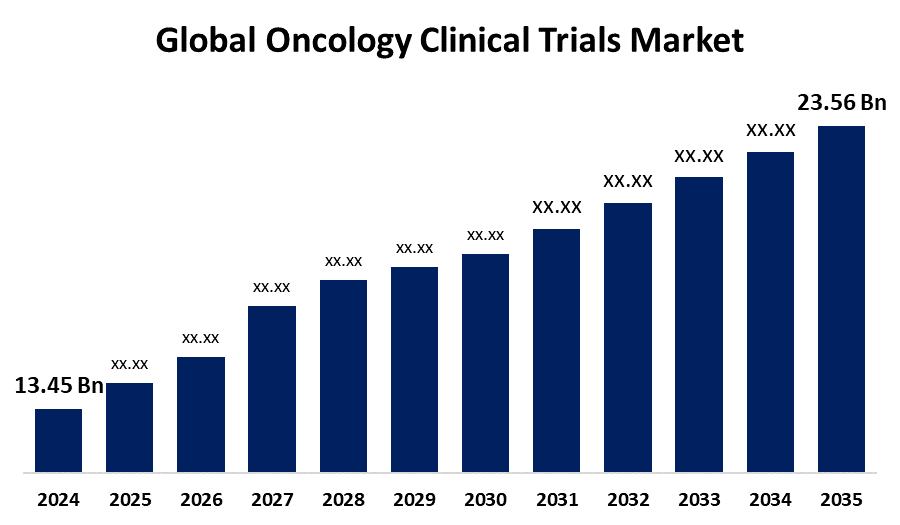
- The Global Oncology Clinical Trials Market Size Was Estimated at USD 13.45 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 5.23% from 2025 to 2035
- The Worldwide Oncology Clinical Trials Market Size is Expected to Reach USD 23.56 Billion by 2035
- Asia Pacific is expected to grow the fastest during the forecast period.
Get more details on this report -
The global oncology clinical trials market size was worth around USD 13.45 billion in 2024 and is predicted to grow to around USD 23.56 billion by 2035 with a compound annual growth rate (CAGR) of 5.23% from 2025 and 2035. The growing incidence of cancer, the need for tailored treatments, technology developments, decentralized trial designs, and the growing investments made by pharmaceutical companies and academic institutions in cutting-edge therapy creation all create opportunities for the oncology clinical trials market.
Market Overview
The global industry dedicated to carrying out regulated clinical research to assess the safety, effectiveness, and results of innovative cancer treatments is known as the oncology clinical trials market. Targeting different cancer types such as lung, breast, and leukemia, it includes trials in all phases (I–IV) with both interventional and observational designs. The market is modified by changing regulatory frameworks and technological integration, and it fosters innovation in immunotherapy, targeted therapy, and cell-based treatments. The increasing need for efficient, individualized oncology solutions around the world is reflected in its expansion.
The market for oncology clinical trials is transforming due to patient-centric trial designs, which prioritize the patient experience and offer substantial chances for efficiency and innovation. The industry is mostly driven by reasons like rising cancer rates, new developments in personalized medicine, and cell and gene therapies, which are launching novel ways to create new treatments for disorders that resemble cancer. The proliferation of oncology clinical trials is fueled by the rising incidence of cancer worldwide.
Report Coverage
This research report categorizes the oncology clinical trials market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the oncology clinical trials market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the oncology clinical trials market.
Global Oncology Clinical Trials Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 13.45 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 5.23% |
| 2035 Value Projection: | USD 23.56 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 225 |
| Tables, Charts & Figures: | 119 |
| Segments covered: | By Phase Type, By Study Design, By Region |
| Companies covered:: | AbbVie Inc., Sanofi, Pivotal, IQVIA Inc, Medpace, Novotech, Astrazeneca, Merck & Co., Inc, Syneos Health, PRA Health Sciences, Gilead Sciences, Inc., Eli Lilly and Company, F. Hoffmann-La Roche Ltd, PAREXEL International Corporation, and Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The market for oncology clinical trials is being driven by an increase in the prevalence of cancer, which also fuels a growth in the demand for a variety of tailored treatment alternatives. The industry is being further supported by the rise in funding for oncology clinical trials from pharmaceutical and biotech firms, non-profits, and CROs. Investing in new treatments, drug development, and therapeutic advancements speeds up clinical research, creating an atmosphere that is conducive to the expansion of the oncology clinical trials market. The market for oncology clinical trials is driven by the rising incidence of cancer worldwide, which greatly increases demand and pushes pharmaceutical companies.
Restraining Factors
The oncology clinical trials market is anticipated to be restricted by social and cultural barriers related to clinical trial participation, recruitment obstacles in the clinical trial process, a lack of scientific knowledge, misuse of statistics and data, and the complexity of the study protocol.
Market Segmentation
The oncology clinical trials market share is classified into phase type and study design.
- The phase I segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period.
Based on the phase type, the oncology clinical trials market is divided into phase I, phase II, phase III, and phase IV. Among these, the phase I segment dominated the market in 2024 and is projected to grow at a substantial CAGR during the forecast period. The oncology clinical trials industry's phase I clinical trial segment is influenced by patient-centered, financial, regulatory, and scientific considerations. A small group of people is tested with an investigational medication or treatment during phase I of clinical trials.
- The interventional studies segment accounted for the largest share in 2024 and is anticipated to grow at a significant CAGR during the forecast period.
Based on the study design, the oncology clinical trials market is divided into interventional studies, observational studies, and expanded access studies. Among these, the interventional studies segment accounted for the largest share in 2024 and is anticipated to grow at a significant CAGR during the forecast period. The special difficulties associated with these circumstances are the main motivating elements for interventional study design in the field of oncology clinical trials. Studies must be carefully planned to increase the likelihood of finding significant treatment effects due to the small patient groups.
Regional Segment Analysis of the Oncology Clinical Trials Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
North America is anticipated to hold the largest share of the oncology clinical trials market over the predicted timeframe.

Get more details on this report -
North America is anticipated to hold the largest share of the oncology clinical trials market over the predicted timeframe. The North America region is known for its high cancer prevalence, strong research ecosystem, and sophisticated healthcare infrastructure. Its prominence is also attributed to a developed pharmaceutical industry and strict regulatory standards. For example, the National Institutes of Health (NIH), a division of the Department of Health and Human Services (HHS), is the largest public funder of biomedical research and development (R&D), according to the U.S. Government Accountability Office. Numerous clinical trials are drawn to North American research institutes and key stakeholders because they actively promote innovation.
Asia Pacific is expected to grow at a rapid CAGR in the oncology clinical trials market during the forecast period. The large and diversified population of the Asia Pacific area offers the chance to recruit a large number of participants, guaranteeing a more representative sample for studies. The need for innovative treatments is highlighted by the region's rising cancer case count. The region's attractiveness for carrying out successful and affordable oncology clinical trials is increased by favorable rules, economical operations, and sophisticated healthcare infrastructure in important countries.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the oncology clinical trials market, along with a comparative evaluation primarily based on their type of offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes type development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- AbbVie Inc.
- Sanofi
- Pivotal
- IQVIA Inc
- Medpace
- Novotech
- Astrazeneca
- Merck & Co., Inc
- Syneos Health
- PRA Health Sciences
- Gilead Sciences, Inc.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd
- PAREXEL International Corporation
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In June 2025, In the Phase 2 clinical trial protocol, which was carried out to assess stenoparib—a differentiated, dual PARP and WNT pathway inhibitor—in patients with recurrent, advanced, platinum-ineligible, or platinum-resistant ovarian cancer, it was announced that the second patient had been dosed. Allarity Therapeutics, Inc., a pharmaceutical business in its Phase 2 clinical stage, developed the stenoparib.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the oncology clinical trials market based on the below-mentioned segments:
Global Oncology Clinical Trials Market, By Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
Global Oncology Clinical Trials Market, By Study Design
- Interventional Studies
- Observational Studies
- Expanded Access Studies
Global Oncology Clinical Trials Market, By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Need help to buy this report?