Global Multi-Cancer Early Detection Market Size, Share, and COVID-19 Impact Analysis, By Type (Liquid Biopsy and Gene Panel, LDT & Others), By End-Use (Hospitals, Diagnostic Laboratories, and Others), By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2022 – 2032.
Industry: HealthcareGlobal Multi-Cancer Early Detection Market Size Insights Forecasts to 2032
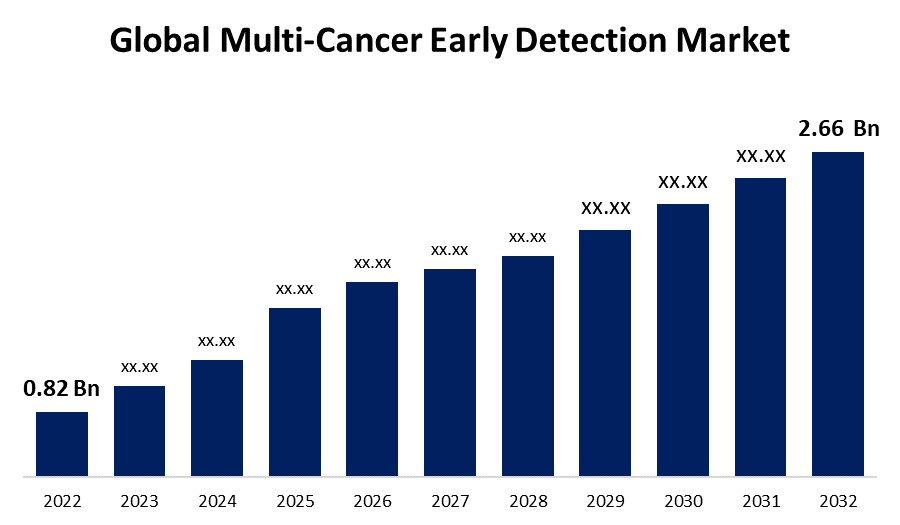
- The Global Multi-Cancer Early Detection Market Size was valued at USD 0.82 Billion in 2022.
- The Market Size is Growing at a CAGR of 12.5% from 2022 to 2032
- The Worldwide Multi-Cancer Early Detection Market Size is expected to reach USD 2.66 Billion by 2032
- North America is expected to Grow higher during the forecast period
Get more details on this report -
The Global Multi-Cancer Early Detection Market Size is expected to reach USD 2.66 Billion by 2032, at a CAGR of 12.5% during the forecast period 2022 to 2032.
Market Overview
Multi-Cancer Early Detection (MCED) is a transformative approach aimed at identifying various cancer types at their initial stages, when treatment outcomes are generally more favorable. By employing advanced techniques such as genomics, proteomics, and liquid biopsies, MCED seeks to detect common molecular signatures and biomarkers shared across multiple cancer types, transcending the limitations of single-cancer screening methods. This comprehensive strategy holds the potential to revolutionize cancer diagnosis by offering a holistic perspective on an individual's health, allowing for timely intervention and personalized treatment plans. However, challenges such as data integration, validation, and ethical considerations must be addressed to ensure the reliability, safety, and equitable access to MCED technologies for widespread clinical implementation.
Report Coverage
This research report categorizes the market for multi-cancer early detection market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the multi-cancer early detection market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segments of the multi-cancer early detection market.
Global Multi-Cancer Early Detection Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2022 |
| Market Size in 2022: | USD 0.82 Billion |
| Forecast Period: | 2022-2032 |
| Forecast Period CAGR 2022-2032 : | 12.5% |
| 2032 Value Projection: | USD 2.66 Billion |
| Historical Data for: | 2018-2021 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 110 |
| Segments covered: | By Type, By End-Use, By Region and COVID-19 Impact Analysis. |
| Companies covered:: | Illumina, Inc., Exact Sciences Corporation, Foundation Medicine, Inc., AnchorDx, Guardant Health, Inc., Burning Rock Biotech Limited, GENECAST, Laboratory for Advanced Medicine, Inc., Singlera Genomics Inc., and other key vendors. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The multi-cancer early detection (MCED) market is propelled by a confluence of critical drivers that collectively contribute to its rapid growth and advancement, the undeniable potential of MCED to revolutionize cancer diagnosis and treatment fuels its expansion. The ability to detect a wide array of cancers at earlier, more treatable stages translates to improved patient outcomes and reduced healthcare costs. Moreover, the advancements in technologies like next-generation sequencing, liquid biopsies, and artificial intelligence have propelled the development of sophisticated MCED platforms, enhancing sensitivity and specificity. The escalating global cancer burden amplifies the urgency for effective early detection methods. As cancer incidence continues to rise, the need for tools capable of screening across multiple cancer types gains significance. MCED's holistic approach addresses this demand by providing a comprehensive diagnostic solution that transcends single-cancer limitations. Increasing investment from both private and public sectors underscores the market's potential. Biotechnology and pharmaceutical companies, as well as research institutions, recognize the value of MCED and channel resources into its research and development. This not only accelerates technological innovations but also fosters partnerships that drive market expansion.
The regulatory agencies' growing recognition of MCED's significance expedites its path to commercialization. Regulatory authorities are working to establish frameworks for the validation and approval of MCED technologies, instilling confidence in clinicians and patients alike. Furthermore, the shift towards personalized medicine further propels MCED adoption. Tailoring treatment plans based on an individual's genetic and molecular profile necessitates comprehensive diagnostic tools like MCED, which can provide a holistic view of a patient's cancer risk and status. Overall, the increasing emphasis on preventive healthcare and wellness further drives MCED's market growth. As individuals become more proactive about their health, there's a rising demand for non-invasive, routine screening approaches that can detect cancer at its earliest stages, enabling timely interventions.
Restraining Factors
Challenges facing the multi-cancer early detection (MCED) market include concerns about the reliability and specificity of identified biomarkers, as false positives could lead to unnecessary invasive procedures. The complexity of interpreting data from multiple cancer types poses analytical challenges. Additionally, the high development costs and lengthy validation processes hinder rapid commercialization. Ethical and privacy considerations regarding genetic information raise apprehensions. Limited awareness among the public and healthcare providers about MCED's benefits and limitations also acts as a restraint.
Market Segmentation
- In 2022, the gene panel, LDT & others segment accounted for around 85.4% market share
On the basis of the type, the global multi-cancer early detection market is segmented into liquid biopsy and gene panel, LDT & others. The gene panel, Laboratory Developed Test (LDT) segment's significant revenue share dominance in the field of multi-cancer early detection (MCED) can be attributed to its pivotal role in personalized medicine. Gene panels offer targeted analysis of multiple genes associated with various cancers, enabling precise risk assessment and early detection. Their versatility in identifying a broad spectrum of cancer-related genetic mutations and alterations drives their adoption across medical settings. Furthermore, the adaptability of LDTs allows researchers and clinicians to tailor panels to specific populations or cancer types, bolstering their utility. This flexibility, coupled with their proven efficacy, establishes gene panel LDTs as a cornerstone in the MCED landscape, resulting in the segment's substantial revenue share.
- The hospitals segment held the largest market with more than 45.2% revenue share in 2022
Based on the end-use, the global multi-cancer early detection market is segmented into hospitals, diagnostic laboratories, and others. The hospitals segment's prominent position as the largest market share holder in multi-cancer early detection (MCED) is primarily due to its role as a central hub for comprehensive healthcare services. Hospitals possess the infrastructure and resources to offer a wide range of medical procedures, including advanced diagnostic techniques like MCED. Their ability to provide integrated care, access to specialized medical professionals, and state-of-the-art equipment enhances the adoption of MCED technologies. Additionally, hospitals' established patient base and referral networks contribute to higher utilization of MCED services. This, in turn, solidifies their dominant position in driving the market for early cancer detection.
Regional Segment Analysis of the Multi-Cancer Early Detection Market
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Rest of APAC)
- South America (Brazil and the Rest of South America)
- The Middle East and Africa (UAE, South Africa, Rest of MEA)
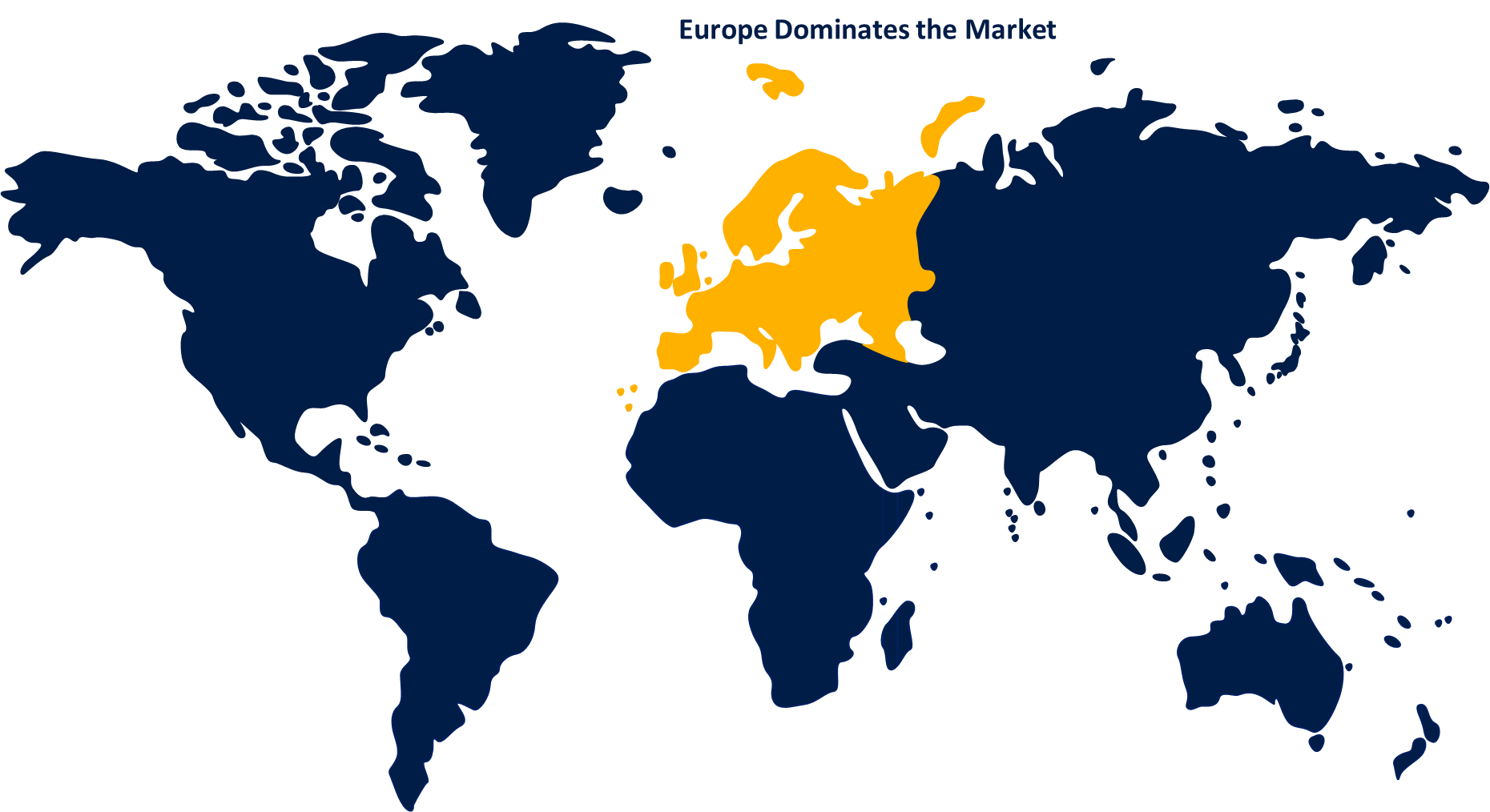
Europe dominated the market with more than 35.2% revenue share in 2022.
Get more details on this report -
Based on region, Europe's dominant market share in multi-cancer early detection (MCED) is attributed to advanced healthcare infrastructure, facilitating the adoption of cutting-edge technologies. Robust research and development initiatives, coupled with substantial investment in biotechnology and healthcare, contribute to the development of innovative MCED solutions. Furthermore, stringent regulatory frameworks ensure high-quality standards and instill confidence in both clinicians and patients. Europe's emphasis on early disease detection and preventive healthcare aligns with the objectives of MCED, driving its popularity. Collectively, these factors establish Europe as a frontrunner in MCED adoption and market expansion.
North America's projected higher growth in the forecast period for multi-cancer early detection (MCED) can be attributed to a strong research and development capabilities, fostering rapid technological advancements in MCED. Access to substantial investment, coupled with a supportive regulatory environment, accelerates innovation and commercialization. Additionally, increasing awareness among both healthcare providers and the public about the potential benefits of early cancer detection drives adoption. The region's emphasis on precision medicine and personalized healthcare aligns with MCED's objectives, positioning North America for accelerated growth in this sector.
Recent Developments
- In April 2022, Elypta, a Swedish molecular diagnostics business, has announced the start of its second clinical trial to investigate the usefulness of GAGomes, or the whole profile of human glycosaminoglycans, as metabolic markers for the early identification of various malignancies (MCED). The purpose of the LEVANTIS-0093A (LEV93A) research is to detect any type of cancer in those who have a high chance of obtaining it due to a lengthy history of smoking.
- In August 2021, GRAIL, a healthcare business specialising in the early detection of some cancers, has been bought by Illumina, Inc.; nevertheless, GRAIL will continue to operate independently while the European Commission performs its ongoing regulatory assessment. GRAIL's Galleri blood test can detect 50 distinct tumours before symptoms occur. With Illumina's acquisition of GRAIL, this potentially life-saving test will become more widely available and used.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global multi-cancer early detection market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Companies:
- Illumina, Inc.
- Exact Sciences Corporation
- Foundation Medicine, Inc.
- AnchorDx
- Guardant Health, Inc.
- Burning Rock Biotech Limited
- GENECAST
- Laboratory for Advanced Medicine, Inc.
- Singlera Genomics Inc.
Key Target Audience
- Market Players
- Investors
- End-Users
- Government Authorities
- Consulting and Research Firm
- Venture Capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at global, regional, and country levels from 2019 to 2032. Spherical Insights has segmented the global multi-cancer early detection market based on the below-mentioned segments:
Multi-Cancer Early Detection Market, By Type
- Liquid Biopsy
- Gene Panel, LDT & Others
Multi-Cancer Early Detection Market, By End-Use
- Hospitals
- Diagnostic Laboratories
- Others
Multi-Cancer Early Detection Market, Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of Middle East & Africa
Need help to buy this report?