Japan Topical Drugs CDMO Market Size, Share, and COVID-19 Impact Analysis, By Product Type (Semi-solid Formulations, Liquid Formulations, Transdermal Products, and Solid Formulations), By Therapeutic Area (Dermatology, Pain Management, Wound Care, Ophthalmology, and Other Therapeutic Areas), By End Use (Pharmaceutical Companies, Biotechnology Companies, and Other End Users), and Japan Topical Drugs CDMO Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Topical Drugs CDMO Market Insights Forecasts to 2035
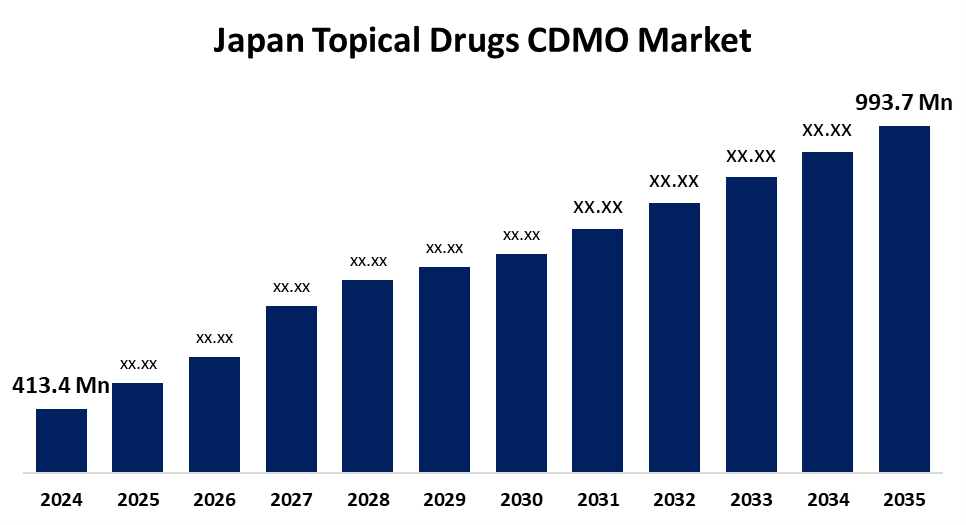
- The Japan Topical Drugs CDMO Market Size Was Estimated at USD 413.4 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 8.3% from 2025 to 2035
- The Japan Topical Drugs CDMO Market Size is Expected to Reach USD 993.7 Million by 2035
Get more details on this report -
According to a Research Report Published by Spherical Insights & Consulting, The Japan Topical Drugs CDMO Market Size is anticipated to Reach USD 993.7 Million by 2035, Growing at a CAGR of 8.3% from 2025 to 2035. The Japan topical drug CDMO market is growing as a result of several factors, among which are the increasing incidence of dermatological disorders, evolving drug delivery technologies, and pharmaceutical manufacturing outsourcing.
Market Overview
The Japan Topical DrugCDMO (Contract Development & Manufaturing Organization) Market Size refers to characterized by contract formulation and manufacturing of semi-solid, liquid, transdermal, and solid dermatological, pain, wound-care, and other topical products. Its motivated by healthcare cost-effectiveness, increasing R&D spending, an aging population, and increasing skin diseases, like acne, psoriasis, and eczema, that need sophisticated targeted delivery systems. Semi-solid products (creams, gels, ointments) are the most prevalent, with solid forms increasing in popularity. Strengths are Japan advanced regulatory framework, CDMOs quality and compliance experise, and simplified approvals, facilitating domestic and international partnerships. Drivers include biosimilar and cell or gene therapy growth, green manufacturing, advanced biologics, and global expansion. Clinical trail reforms and the emerging biosimilars and cell or gene therapy markets also boost the need for outsourcing. Market entry is facilitated by government initiatives, including regulatory simplification through PMDA, fast-track programs for regenerative medicines, and global harmonization.
Report Coverage
This research report categorizes the market for the Japan topical drugs CDMO market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan topical drugs CDMO market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan topical drugs CDMO market.
Japan Topical Drugs CDMO Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 413.4 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 8.3% |
| 2035 Value Projection: | USD 993.7 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 203 |
| Tables, Charts & Figures: | 92 |
| Segments covered: | By Product Type, By Therapeutic Area, By End Use |
| Companies covered:: | Takeda Pharmaceutical, Johnson & Johnson, Teva Pharmaceuticals, Novartis AG, Sun Pharma Japan Limited, Hisamitsu Pharmaceutical, GlaxoSmithKline plc, Nichi-Iko Pharmaceutical Co., Ltd., Daiichi Sankyo, Nipro Corporation, Mitsubishi Tanabe Pharma, Meiji Seika Pharma, and Other Key Companies. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The Japan topical drugs CDMO market is fueled by increasing demand for dermatological therapies, an aging population, and a growing incidence of chronic skin disorders such as eczema and psoriasis. Cost savings and time-to-market acceleration are driving companies to outsource to pharmaceutical manufacturers. Japans robust regulatory environment, high manufacturing standards, and experience in complex formulations drive CDMO expansion. Technological innovations in drug delivery technologies and government facilitation also propel the market upwards.
Restraining Factors
Restraining factors for Japans topical drugs CDMO market are high labor and operational costs, strict regulatory regulations, limited scalability for manufacturing of niche products, and fierce competition from Asia-based low-cost CDMOs, which challenge profitability and worldwide competitiveness.
Market Segmentation
The Japan topical drugs CDMO market share is classified into product type, therapeutic area, and end use.
- The semi-solid formulations segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan topical drugs CDMO market is segmented by product type into semi-solid formulations, liquid formulations, transdermal products, and solid formulations. Among these, the semi-solid formulations segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to their good acceptance for the treatment of various skin conditions. These products deliver targeted API to the lesion while keeping the active ingredient system circulation at a minimum, which makes them suitable for dermatological use.
- The dermatology segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan topical drugs CDMO market is segmented by therapeutic area into dermatology, pain management, wound care, ophthalmology, and other therapeutic areas. Among these, the dermatology segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is owing to the tremendous burden of skin diseases and the increasing demand for good topical treatment options. Over one hundred million individuals worldwide have skin diseases such as acne, psoriasis, eczema, and skin infections. This has prompted pharmaceutical manufacturers to come up with topical formulation drugs that will curb these issues.
- The pharmaceutical companies segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan topical drugs CDMO market is segmented by end use into pharmaceutical companies, biotechnology companies, and other end-users. Among these, the pharmaceutical companies segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is attributed to their growing dependence on outsourcing in drug development and production. These companies are making their drug pipelines stronger to keep pace with the rising incidence rate of skin diseases, which in turn will increase the demand for topical treatments.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan topical drugs CDMO market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Takeda Pharmaceutical
- Johnson & Johnson
- Teva Pharmaceuticals
- Novartis AG
- Sun Pharma Japan Limited
- Hisamitsu Pharmaceutical
- GlaxoSmithKline plc
- Nichi-Iko Pharmaceutical Co., Ltd.
- Daiichi Sankyo
- Nipro Corporation
- Mitsubishi Tanabe Pharma
- Meiji Seika Pharma
- Others
Recent Developments:
- In June 2024, Takeda announced that LIVTENCITY® (maribavir) has been approved in Japan by the Japanese Ministry of Health, Labour and Welfare (MHLW) for post-transplant cytomegalovirus (CMV) infection/disease refractory to other anti-CMV therapies. LIVTENCITY is the first and sole post-transplant anti-CMV therapy approved in Japan that acts through the inhibition of pUL97 kinase and its natural substrates.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan topical drugs CDMO market based on the below-mentioned segments:
Japan Topical Drugs CDMO Market, By Product Type
- Semi-solid Formulations
- Liquid Formulations
- Transdermal Products
- Solid Formulations
Japan Topical Drugs CDMO Market, By Therapeutic Area
- Dermatology
- Pain Management
- Wound Care
- Ophthalmology
- Other Therapeutic Areas
Japan Topical Drugs CDMO Market, By End Use
- Pharmaceutical Companies
- Biotechnology Companies
- Other End Users
Need help to buy this report?