Japan Sterility Testing Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Rapid Sterility Tests and Conventional Sterility Tests), By Product Type (Instruments, Kits & Reagents, and Others), By Application (Medical Device Manufacturing, Biopharmaceutical Manufacturing, and Others), and Japan Sterility Testing Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Sterility Testing Market Insights Forecasts to 2035
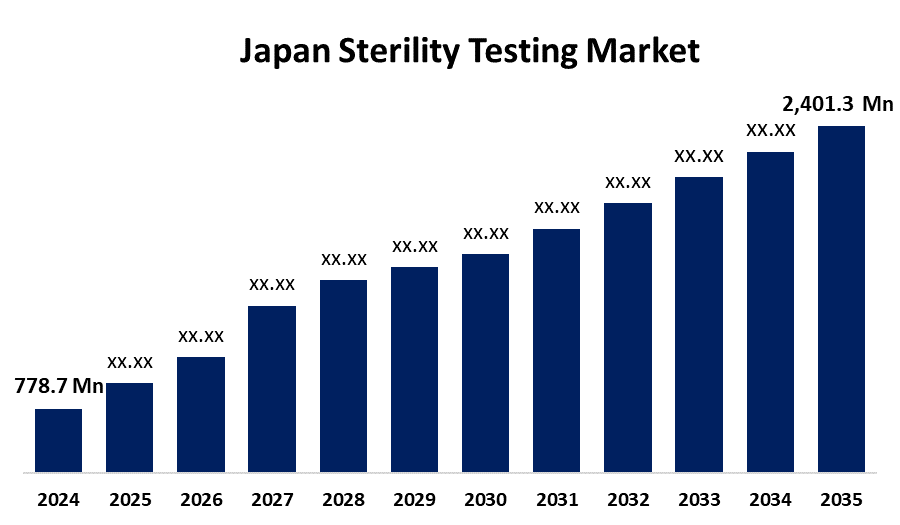
- The Japan Sterility Testing Market Size Was Estimated at USD 778.7 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 10.78% from 2025 to 2035
- The Japan Sterility Testing Market Size is Expected to Reach USD 2,401.3 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the Japan sterility testing market is anticipated to reach USD 2,401.3 million by 2035, growing at a CAGR of 10.78% from 2025 to 2035. The growth of the Japan sterility testing market is due to technological advancements, the need for biologics and vaccines, and stringent regulatory conditions in the pharmaceutical industry. In addition, increased healthcare spending and the need for pharmaceutical manufacturing quality control are drivers of the growth of the market.
Market Overview
The Japan sterility testing market refers to the pharmaceutical product safety and performance, particularly with the aging population and increasing incidence of chronic diseases in Japan. Sterility testing is mandatory for all pharmaceutical products at the time of release, in line with Japan's stringent regulatory requirements. Japan's advanced technology environment and adherence to international standards of quality further the credibility and effectiveness of sterility testing processes. The potential in using and implementing rapid microbial methods (RMMs) and outsourcing sterility testing to contract research organizations (CROs) that have expertise in such a procedure, which can be streamlined at a lower cost of operation. They also include the rise in demand for safe drugs, the rise in the consumption of biopharmaceuticals, and the rise in demand for bacterial endotoxin testing. The Pharmaceuticals and Medical Devices Agency (PMDA) is vital in ensuring the safety, efficacy, and quality of medicines and medical devices in Japan.
Report Coverage
This research report categorizes the market for the Japan sterility testing market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan sterility testing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan sterility testing market.
Japan Sterility Testing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 778.7 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 10.78% |
| 2035 Value Projection: | USD 2,401.3 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 215 |
| Tables, Charts & Figures: | 115 |
| Segments covered: | By Test Type, By Product Type, By Application and COVID-19 Impact Analysis |
| Companies covered:: | Fujirebio, Synergy Health, Danaher Corporation, Shimadzu Corporation, Merck KGaA, BioMérieux, Shin-Etsu Chemical Co., Ltd., Charles River Laboratories, WuXi AppTec, Thermo Fisher Scientific, Sartorius AG, Japan Food Research Laboratories (JFRL), Thermo Fisher Scientific, Others. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis. |
Get more details on this report -
Driving Factors
The Japan sterility testing market is driven by increasing pharmaceutical production, biopharmaceutical requirements, and aging populations requiring sterile medications. Stringent regulatory conditions by bodies such as the PMDA ensure stringent quality requirements. Increased focus on patient protection and post-pandemic focus on contamination control increases demand. In addition, advances in rapid tests of microbial content assure better sterility assurance in the pharmaceutical and medical device production industries.
Restraining Factors
Japan sterility testing market is constrained by the high cost of procedures, the absence of professional expertise, and the difficulty in achieving an aseptic environment for the test. Complicated regulations and stringent compliance demands might also hamper the growth of the market. All these challenges combined create operational inefficiency and increased costs, which might hinder the adoption of the sterility test in Japan across sectors.
Market Segmentation
The Japan sterility testing market share is classified into test type, product type, and application.
- The conventional sterility tests segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan sterility testing market is segmented by test type into rapid sterility tests and conventional sterility tests. Among these, conventional sterility tests segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to the low cost of setup and return on investment in the short term. Sterility tests are traditionally also classified into membrane filtration and immersion tests.
- The kits & reagents segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan sterility testing market is segmented by product type into instruments, kits & reagents, and others. Among these, the kits & reagents segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to the requirement of repeated purchasing, product affordability, and ongoing research & development work. Reagents and kits are a fundamental part of the procedure of sterility testing and are used in sample preparation, culture medium preparation, and identification of microorganisms.
- The biopharmaceutical manufacturing segment dominated the market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan sterility testing market is segmented by application into medical device manufacturing, biopharmaceutical manufacturing, and others. Among these, the biopharmaceutical manufacturing segment dominated the market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is attributed to ongoing research and development of numerous chronic and life-threatening illnesses. The growing demand for biopharmaceuticals as a result of the growth in biotechnology and the rising prevalence of chronic diseases underscores the essential role of sterility testing in ensuring the safety and efficacy of these advanced therapeutic products.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan sterility testing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Fujirebio
- Synergy Health
- Danaher Corporation
- Shimadzu Corporation
- Merck KGaA
- BioMérieux
- Shin-Etsu Chemical Co., Ltd.
- Charles River Laboratories
- WuXi AppTec
- Thermo Fisher Scientific
- Sartorius AG
- Japan Food Research Laboratories (JFRL)
- Thermo Fisher Scientific
- Others
Recent Developments:
- In September 2023, Companies like Synergy Health and BioMérieux have extended their product lines to address the growing demand for quality sterility testing solutions during increased quality control measures in pharmaceutical production.
- In August 2023, Danaher Corporation announced its plans to buy a prominent sterilization technology company that will further strengthen it in this business segment.
- In July 2023, WuXi AppTec formed a partnership with Lonza for the co-development of new sterility test guidelines specifically for the Japanese market.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan sterility testing market based on the below-mentioned segments:
Japan Sterility Testing Market, By Test Type
- Rapid Sterility Tests
- Conventional Sterility Tests
Japan Sterility Testing Market, By Product Type
- Instruments
- Kits & Reagents
- Others
Japan Sterility Testing Market, By Application
- Medical Device Manufacturing
- Biopharmaceutical Manufacturing
- Others
Need help to buy this report?