Japan Recombinant Proteins Manufacturing Services Market Size, Share, and COVID-19 Impact Analysis, By Service Type (Pre-clinical & Clinical Services, and Commercial Production Services), By Host Cell (Mammalian Cells, Bacterial Cells, Insect Cells, Yeast & Fungi, and Others), By End-user (Pharmaceutical & Biotechnology Companies, and Academic & Research Institutes), and Japan Recombinant Proteins Manufacturing Services Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Recombinant Proteins Manufacturing Services Market Insights Forecasts to 2035
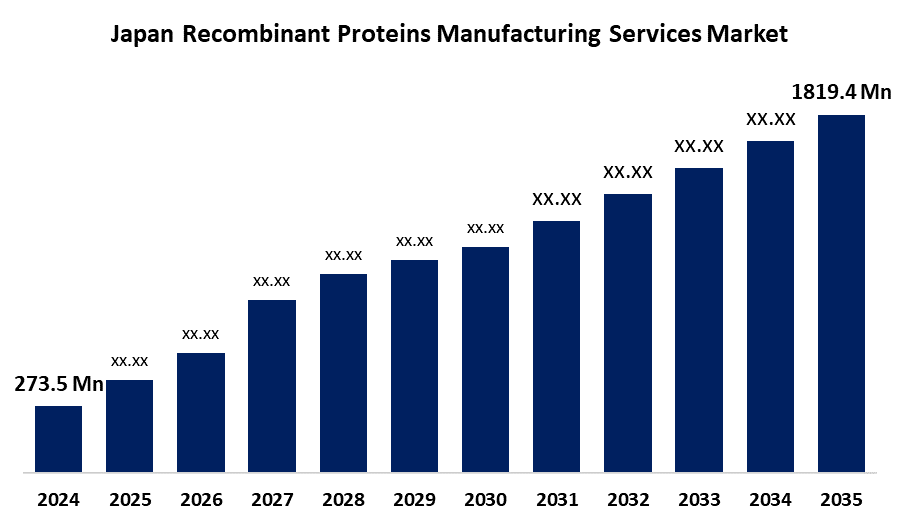
- The Japan Recombinant Proteins Manufacturing Services Market Size Was Estimated at USD 273.5 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 18.8% from 2025 to 2035
- The Japan Recombinant Proteins Manufacturing Services Market Size is Expected to Reach USD 1819.4 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, The Japan Recombinant Proteins Manufacturing Services Market Size is anticipated to reach USD 1819.4 Million by 2035, growing at a CAGR of 18.8% from 2025 to 2035. The Japan recombinant proteins manufacturing services market is increasing due to increasing chronic diseases, improvements in genetic engineering, and higher R&D activities, all driving demand for recombinant proteins in treatment and research purposes in a range of industries.
Market Overview
The Japan recombinant proteins manufacturing services market refers to the outsourced manufacturing of proteins such as cytokines, growth factors, antibodies, and hormones utilized in drug discovery, biopharma therapeutic development, diagnostics, and academic studies. It consists of both commercial manufacturing services and turnkey pre-clinical or clinical services. Japan competencies are superior biotechnology infrastructure, mammalian cell-based expression system expertise, and mature protein expression platforms in both prokaryotic and eukaryotic hosts. New opportunities arise from expansion in pre-clinical and clinical stage services, growth hormone and cytokine manufacturing, and academic partnerships. Market drivers are robust R&D spending by pharmaceutical and biotech companies, increasing demand for precision-targeted biologics, and the shift towards specialized CDMO outsourcing. Initiatives by the government, such as public and private councils for foreign investment captivation, drug discovery encouragement strategies, and abbreviated regulatory routes such as the Sakigake advance designation, also reinforce biotech ecosystem growth in Japan.
Report Coverage
This research report categorizes the market for the Japan recombinant proteins manufacturing services market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan recombinant proteins manufacturing services market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan recombinant proteins manufacturing services market.
Japan Recombinant Proteins Manufacturing Services Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 273.5 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 18.8% |
| 2035 Value Projection: | USD 1819.4 million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 213 |
| Tables, Charts & Figures: | 123 |
| Segments covered: | By Service Type, By Host Cell and By End-user |
| Companies covered:: | Lonza Group, Fujifilm Holdings Corporation, Takara Bio Inc., Kaneka Corporation, PeptiDream Inc., Synbio Technologies, Merck KGaA, CellFree Sciences Co., Ltd., Sino Biological, Ajinomoto Bio Pharma Services, Bio Rad Laboratories, Inc., GenScript Biotech Corporation, and Others |
| Pitfalls & Challenges: | COVID-19 Impact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The Japan recombinant proteins manufacturing services market is propelled by the growing R&D expenditures of pharmaceutical and biotechnology firms, escalating needs for biologics and targeted therapies, and the increasing outsourcing of protein production to dedicated CDMOs. The growing average price per vial for recombinant proteins also fuels the growth of the market. Moreover, the advances in expression systems and bioprocessing technologies, along with government support and an extensive academic industry network, further drive demand for quality, scalable protein manufacturing services.
Restraining Factors
High production costs, intricate regulatory frameworks, and long approval times pose threats to the Japan recombinant proteins manufacturing services market. Shortage of skilled resources and competition from affordable manufacturing nations also limit market expansion and project timelines.
Market Segmentation
The Japan recombinant proteins manufacturing services market share is classified into service type, host cell, and end-user.
- The commercial production services segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan recombinant proteins manufacturing services market is segmented by service type into pre clinical & clinical services, and commercial production services. Among these, the commercial production services segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to they are required to meet stringent regulations such as Good Manufacturing Practices (GMP) to provide safety, efficacy, and consistency, which demands sophisticated expertise and firm, certified production processes. Large scale manufacturing of recombinant proteins for clinical use and commercial purposes is managed by commercial production services, which have high demand.
- The mammalian cells segment dominated the market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan recombinant proteins manufacturing services market is segmented by host cell into mammalian cells, bacterial cells, insect cells, yeast & fungi, and others. Among these, the mammalian cells segment dominated the market share in 2024 and is expected to grow at a significant CAGR during the forecast period. Mammalian cell-based systems generate recombinant proteins with very close similarity to natural human proteins with correct folding and post-translational modifications that are crucial for biopharmaceutical activity. The systems have a certified record of safety, and regulatory authorities such as the FDA and EMA prefer them because of their consistency and minimal chances of contamination.
- The pharmaceutical & biotechnology companies segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan recombinant proteins manufacturing services market is segmented by end-user into pharmaceutical & biotechnology companies, and academic & research institutes. Among these, the pharmaceutical & biotechnology companies segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is attributed to their strong R&D activities and the therapeutic development demand for biologics. Their focus on commercial-scale and clinical trial production generates substantial market share over research and academic institutes.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan recombinant proteins manufacturing services market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Lonza Group
- Fujifilm Holdings Corporation
- Takara Bio Inc.
- Kaneka Corporation
- PeptiDream Inc.
- Synbio Technologies
- Merck KGaA
- CellFree Sciences Co., Ltd.
- Sino Biological
- Ajinomoto Bio Pharma Services
- Bio Rad Laboratories, Inc.
- GenScript Biotech Corporation
- Others
Recent Developments:
- In January 2023, Kaneka Corporation is expanding GMP manufacturing for mRNA at its European subsidiary, Kaneka Eurogentec, with a 2 billion JPY investment, increasing capacity fivefold by late 2023. This expansion meets rising worldwide demand for mRNA in vaccines and therapeutics for infectious, genetic, and cancer diseases.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan recombinant proteins manufacturing services market based on the below-mentioned segments:
Japan Recombinant Proteins Manufacturing Services Market, By Service Type
- Pre-clinical & Clinical Services
- Commercial Production Services
Japan Recombinant Proteins Manufacturing Services Market, By Host Cell
- Mammalian Cells
- Bacterial Cells
- Insect Cells
- Yeast & Fungi
- Others
Japan Recombinant Proteins Manufacturing Services Market, By End-user
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
Need help to buy this report?