Japan Point of Care Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Product (Infectious Disease Testing Products, Blood Glucose Products, and Blood Gas & Electrolyte Products), By End User (Hospitals & Clinics, Diagnostic Laboratories, and Home Care Settings), and Japan Point of Care Diagnostics Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Point of Care Diagnostics Market Insights Forecasts to 2035
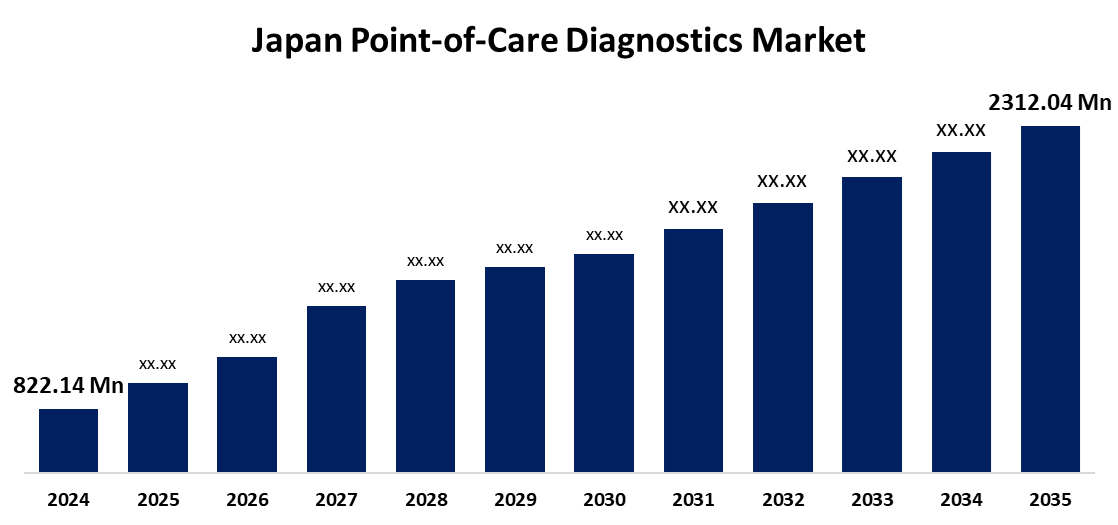
- The Japan Point of Care Diagnostics Market Size Was Estimated at USD 822.14 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 9.86% from 2025 to 2035
- The Japan Point of Care Diagnostics Market Size is Expected to Reach USD 2312.04 Million by 2035
Get more details on this report -
According to a Research Report Published by Spherical Insights & Consulting, The Japan Point of Care Diagnostics Market Size is anticipated to Reach USD 2312.04 Million by 2035, Growing at a CAGR of 9.86% from 2025 to 2035. The growth of Japans POCD market is primarily fueled by the rising prevalence of chronic diseases such as diabetes, cardiovascular disorders, and cancer, especially among the aging population.
Market Overview
Point of care diagnostics (POCD) refers to medical testing conducted at or near the site of patient care, enabling rapid clinical decisions without the need for centralized laboratories. These tests are designed to be user friendly, require minimal training, and cover a wide range of conditions such as infectious diseases, chronic illnesses, and pregnancy. In Japan, POCD is increasingly integrated into community clinics, pharmacies, and home care settings, enhancing accessibility and reducing healthcare system burdens. The market includes technologies like lateral flow assays, molecular diagnostics, and microfluidics. Additionally, government initiatives promoting healthcare modernization, favorable reimbursement policies, and strong R&D investments are accelerating adoption.
Report Coverage
This research report categorizes the market for the Japan point of care diagnostics market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan point of care diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub segment of the Japan point of care diagnostics market.
Japan Point of Care Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 822.14 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 9.86% |
| 2035 Value Projection: | USD 2312.04 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 190 |
| Tables, Charts & Figures: | 95 |
| Segments covered: | By Product, By End User |
| Companies covered:: | Becton, Dickinson and Company (BD), Quidel Corporation, QIAGEN, Trinity Biotech, BioMérieux SA, and Other Key Companies. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The quick development of diagnostic technologies is one of the main factors propelling the point of care diagnostics (POCD) industry in Japan. In order to make POCD equipment more precise, easy to use, and able to handle more complicated diagnostic tasks, technological innovation has been crucial as healthcare systems move toward speedier, more decentralized care. Japan has been in the forefront of creating small, effective diagnostic tools that can provide trustworthy results at or close to the point of patient care. Japan is renowned for its expertise in electronics, robotics, and precision engineering. Portable, handheld gadgets that offer real time data in both clinical and non clinical situations have become possible due to the downsizing of diagnostic equipment.
Restraining Factors
The strict and intricate regulations governing medical devices and in vitro diagnostics (IVDs) in Japan are one of the main factors impeding the expansion of the point of care diagnostics (POCD) market in that nation. Before being put on the market in Japan, all medical products and diagnostics—including POCD tools—must meet the standards established by the Pharmaceuticals and Medical products Agency (PMDA) and be approved by the Ministry of Health, Labour, and Welfare (MHLW).
Market Segmentation
The Japan point of care diagnostics market share is classified into product and end-user.
- The infectious disease testing products segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan point of care diagnostics market is segmented by product into infectious disease testing products, blood glucose products, and blood gas & electrolyte products. Among these, the infectious disease testing products segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. The early detection and control of infectious diseases are greatly aided by infectious disease testing products. The purpose of these diagnostic instruments is to identify pathogens, including bacteria, viruses, and other microbes, directly from patient samples.
- The hospitals & clinics segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan point of care diagnostics market is segmented by end user into hospitals & clinics, diagnostic laboratories, and home care settings. Among these, the hospitals & clinics segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. This dominance is primarily due to Japans advanced healthcare infrastructure and the central role hospitals and clinics play in delivering diagnostic services.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan point of care diagnostics market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Becton
- Dickinson and Company (BD)
- Quidel Corporation
- QIAGEN
- Trinity Biotech
- BioMérieux SA
- Others
Key Target Audience
- Market Players
- Investors
- End users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan Point of care diagnostics Market based on the following segments:
Japan Point of Care Diagnostics Market, By Product
- Infectious Disease Testing Products
- Blood Glucose Products
- Blood Gas & Electrolyte Products
Japan Point of Care Diagnostics Market, By End User
- Hospitals & Clinics
- Diagnostic Laboratories
- Home Care Settings
Need help to buy this report?