Japan Orphan Drugs Market Size, Share, and COVID-19 Impact Analysis, By Disease Type (Oncological, Metabolic, Hematologic & Immunologic, Infectious, and Neurological), By Indication (Non-Hodgkin Lymphoma, Acute Myeloid Leukemia, Cystic Fibrosis, Glioma, Pancreatic Cancer, Ovarian Cancer, Multiple Myeloma, Duchenne Muscular Dystrophy, Graft vs Host Disease, and Renal Cell Carcinoma), and Japan Orphan Drugs Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareJapan Orphan Drugs Market Insights Forecasts to 2033
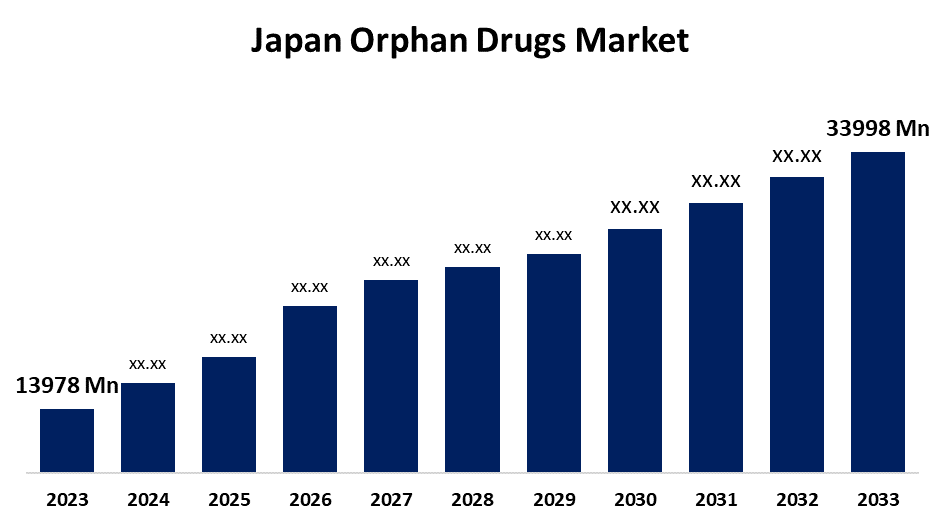
- The Japan Orphan Drugs Market Size was Valued at USD 13978 Million in 2023.
- The Market is Growing at a CAGR of 9.30% from 2023 to 2033
- The Japan Orphan Drugs Market Size is Expected to Reach USD 33998 Million by 2033
Get more details on this report -
The Japan Orphan Drugs Market Size is Anticipated to Reach USD 33998 Million by 2033, Growing at a CAGR of 9.30% from 2023 to 2033.
Market Overview
Japan orphan drugs market refers to the segment of the pharmaceutical industry that focuses on the development, production, and distribution of drugs for rare diseases, often referred to as orphan diseases. In Japan, orphan drugs are regulated and incentivized under specific frameworks designed to encourage innovation in this area, addressing the unmet medical needs of patients with rare conditions. The Japanese government has established a range of measures, including the Orphan Drug Act, which offers various incentives such as tax reductions, subsidies for clinical trials, and extended market exclusivity. Additionally, the Pharmaceutical and Medical Devices Agency (PMDA) facilitates the fast-track approval process for orphan drugs, ensuring that these treatments reach patients more quickly than standard drugs. For the pharmaceutical industry, these incentives make the development of orphan drugs more financially feasible, which can lead to increased assets in innovation. Moreover, the market for orphan drugs in Japan is growing rapidly due to an aging population, which increases the prevalence of rare diseases and the demand for specialized treatments.
Report Coverage
This research report categorizes the market for the Japan orphan drugs based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan orphan drugs market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan orphan drugs market.
Japan Orphan Drugs Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 13978 Million |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 9.30% |
| 2033 Value Projection: | USD 33998 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 180 |
| Tables, Charts & Figures: | 130 |
| Segments covered: | By Disease Type, By Indication and COVID-19 Impact Analysis. |
| Companies covered:: | Shionogi & Co., Ltd., Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Daiichi Sankyo Company, Limited, Eisai Co., Ltd., Pfizer, Inc., Astellas Pharma Inc., Merck KGaA, Takeda Pharmaceutical Company Limited, and Others key Vendors. |
| Pitfalls & Challenges: | COVID-19 Impact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The elderly population in Japan is a significant catalyst. As the elderly population increases, the incidence of age-related rare diseases, such as neurodegenerative conditions, is on the rise, creating greater demand for specialized treatments. Additionally, advancements in medical research and biotechnology are enabling the development of more effective orphan drugs. Furthermore, the growing awareness and advocacy around rare diseases are helping to drive demand. As patient organizations and advocacy groups raise awareness about the needs of those suffering from rare conditions, public pressure is building for better access to treatments.
Restraining Factors
One of the main obstacles is the high cost of research and development (R&D) associated with orphan drugs.
Market Segmentation
The Japan orphan drugs market share is classified into disease type and indication.
- The oncological segment accounted for the leading revenue share in 2023 and is expected to grow at a significant CAGR during the forecast period.
The Japan orphan drugs market is segmented by disease type into oncological, metabolic, hematologic & immunologic, infectious, and neurological. Among these, the oncological segment accounted for the leading revenue share in 2023 and is expected to grow at a significant CAGR during the forecast period. The segment growth is driven by substantial advancements in cancer therapies, including targeted treatments, immunotherapies, and gene therapies. These innovations, coupled with strong regulatory support and an increasing focus on rare cancers, are expected to continue propelling growth in the oncology segment.
- The non-Hodgkin lymphoma segment accounted for a significant market share in 2023 and is expected to grow at a substantial CAGR during the forecast period.
The Japan orphan drugs market is segmented by indication into non-hodgkin lymphoma, acute myeloid leukemia, cystic fibrosis, glioma, pancreatic cancer, ovarian cancer, multiple myeloma, Duchenne muscular dystrophy, graft vs host disease, and renal cell carcinoma. Among these, the non-Hodgkin lymphoma segment accounted for a significant market share in 2023 and is expected to grow at a substantial CAGR during the forecast period. The growth can be attributed to several factors, including the increasing prevalence of NHL, advancements in targeted therapies such as monoclonal antibodies and CAR-T cell therapies, and a growing emphasis on precision medicine.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan orphan drugs market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Shionogi & Co., Ltd.
- Chugai Pharmaceutical Co., Ltd.
- Otsuka Pharmaceutical Co., Ltd.
- Kyowa Hakko Kirin Co., Ltd.
- Daiichi Sankyo Company
- Limited, Eisai Co., Ltd.
- Pfizer, Inc.
- Astellas Pharma Inc.
- Merck KGaA
- Takeda Pharmaceutical Company Limited
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Japan orphan drugs market based on the below-mentioned segments:
Japan Orphan Drugs Market, By Disease Type
- Oncological
- Metabolic
- Hematologic & Immunologic
- Infectious
- Neurological
Japan Orphan Drugs Market, By Indication
- Non-Hodgkin Lymphoma
- Acute Myeloid Leukemia
- Cystic Fibrosis
- Glioma
- Pancreatic Cancer
- Ovarian Cancer
- Multiple Myeloma
- Duchenne Muscular Dystrophy
- Graft vs Host Disease
- Renal Cell Carcinoma
Need help to buy this report?