Japan Mycoplasma Testing Market Size, Share, and COVID-19 Impact Analysis, By Product (Instruments, Kits & Reagents, and Services), By Technology (PCR, ELISA, Direct Assay, Indirect Assay, Microbial Culture Techniques, and Enzymatic Methods), By Application (Cell Line Testing, Virus Testing, End of Production Cells Testing, and Others), and Japan Mycoplasma Testing Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Mycoplasma Testing Market Insights Forecasts to 2035
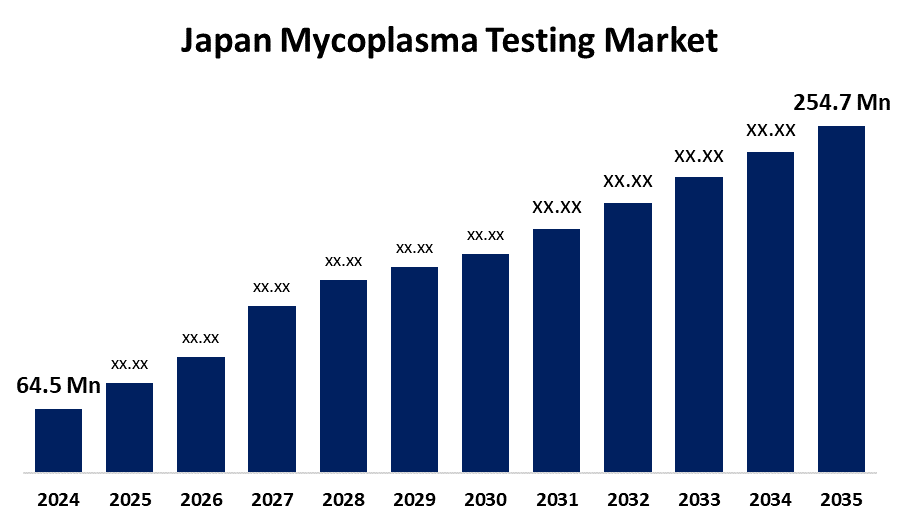
- The Japan Mycoplasma Testing Market Size Was Estimated at USD 64.5 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 13.3% from 2025 to 2035
- The Japan Mycoplasma Testing Market Size is Expected to Reach USD 254.7 Million by 2035
Get more details on this report -
According to a Research Report Published by Spherical Insights & Consulting, The Japan Mycoplasma Testing Market Size is anticipated to Reach USD 254.7 Million by 2035, Growing at a CAGR of 13.3% from 2025 to 2035. The Japan mycoplasma testing market is growing with the increasing biopharmaceutical R&D, increasing demand for biologics and vaccines, and stringent regulatory requirements. Advances in technology, such as PCR and DNA staining, also improve testing efficiency and accuracy, increasing adoption across research and production industries.
Market Overview
The Japan Mycoplasma Testing Market Size refers to diagnostic kits, instruments, and services that are used to identify contamination in cell cultures, biologics, and vaccines. Extensively deployed in the pharmaceutical, biotechnology, and research industries, it provides product safety and regulatory support. Strengths are Japan sophisticated biotech facilities, sound industry academic partnerships, and rising attention to contamination threats. Opportunities are in serving expanding markets such as cell and gene therapy, contract research organizations, and vaccine production, all of which require rigorous contamination control. The market is driven considerably by growth in biopharmaceutical R&D and increased demand for biosimilars and vaccines. Government backing through regulatory focus on quality standards and biosafety, and targeted financing for biotech innovation, also drives market growth.
Report Coverage
This research report categorizes the market for the Japan mycoplasma testing market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan mycoplasma testing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan mycoplasma testing market.
Japan Mycoplasma Testing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 64.5 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 13.3% |
| 2035 Value Projection: | USD 254.7 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 190 |
| Tables, Charts & Figures: | 98 |
| Segments covered: | By Product, By Technology |
| Companies covered:: | Nissui Pharmaceutical Co., Ltd., Nipro Corporation, Sysmex Corporation, JEOL Ltd., Charles River Laboratories, Tosoh Corporation, Kyokuto Pharmaceutical Industrial Co., Ltd., COSMO BIO Co., Ltd., Asahi Kasei Corporation, Techno Suruga Laboratory Co., Ltd., Lonza Group Ltd., Merck KGaA, Thermo Fisher Scientific, Inc., and Other Key Companies. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The Japan mycoplasma testing market is spurred by the high growth of the biopharmaceutical and biotechnology industries, rising production of biologics and vaccines, and expanding concern over contamination risk. High regulatory standards of agencies such as the PMDA drive demand for trustworthy testing. Technological advancements in molecular diagnostic tools, including PCR and ELISA, improve the efficiency and accuracy of detection. Rising need for cell and gene therapies and biosimilars also contributes to market growth, along with government encouragement of life sciences innovation.
Restraining Factors
The Japan mycoplasma testing market is hindered by the high price of sophisticated testing instrumentation and methodologies, restricting use for smaller labs. Inconsistent and stringent regulatory demands raise compliance costs, while the lack of experienced professionals delays deployment and consistent application of advanced testing technologies.
Market Segmentation
The Japan mycoplasma testing market share is classified into product, technology, and application.
- The kits & reagents segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan mycoplasma testing market is segmented by product into instruments, kits & reagents, and services. Among these, the kits & reagents segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to the increasing demand for rapid, sensitive, and cost-effective mycoplasma detection in biopharmaceutical manufacturing, CROs, and universities is propelling market expansion. PCR- and ELISA-based kit technologies provide quick, reliable results that more effectively meet cell culture and biologics manufacturing contamination risks than time-consuming, labor-intensive conventional culture techniques.
- The PCR segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan mycoplasma testing market is segmented by technology into PCR, ELISA, direct assay, indirect assay, microbial culture techniques, and enzymatic methods. Among these, the PCR segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. PCR-based mycoplasma analysis is very sensitive, quick, and superior to conventional testing. It identifies minute contamination of cell cultures, biopharmaceuticals, and gene therapy vectors in a matter of hours, as opposed to the weeks involved for culture tests, making it the first choice of pharmaceutical, biotech companies, CROs, and research organizations.
- The cell line testing segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan mycoplasma testing market is segmented by application into cell line testing, virus testing, end of production cells testing, and others. Among these, the cell line testing segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. Cell lines are critical in biopharmaceutical production and research, but mycoplasma contamination can harm research integrity and product safety. To meet GMP and FDA, EMA, and ICH regulations, regular mycoplasma testing must be undertaken, particularly with increasing cell-based assays, monoclonal antibodies, and gene therapy uses.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan mycoplasma testing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Nissui Pharmaceutical Co., Ltd.
- Nipro Corporation
- Sysmex Corporation
- JEOL Ltd.
- Charles River Laboratories
- Tosoh Corporation
- Kyokuto Pharmaceutical Industrial Co., Ltd.
- COSMO BIO Co., Ltd.
- Asahi Kasei Corporation
- Techno Suruga Laboratory Co., Ltd.
- Lonza Group Ltd.
- Merck KGaA
- Thermo Fisher Scientific, Inc.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan mycoplasma testing market based on the below-mentioned segments:
Japan Mycoplasma Testing Market, By Product
- Instruments
- Kits & Reagents
- Services
Japan Mycoplasma Testing Market, By Technology
- PCR
- ELISA
- Direct Assay
- Indirect Assay
- Microbial Culture Techniques
- Enzymatic Methods
Japan Mycoplasma Testing Market, By Application
- Cell Line Testing
- Virus Testing
- End of Production Cells Testing
- Others
Need help to buy this report?