Japan Medical Device Contract Manufacturing Market Size, Share, and COVID-19 Impact Analysis, By Type (Class I, Class II, and Class III), By Source (Accessories Manufacturing, Assembly, Component Manufacturing, Device Manufacturing, Packaging and Labelling, and Other), and Japan Medical Device Contract Manufacturing Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Medical Device Contract Manufacturing Market Insights Forecasts to 2035
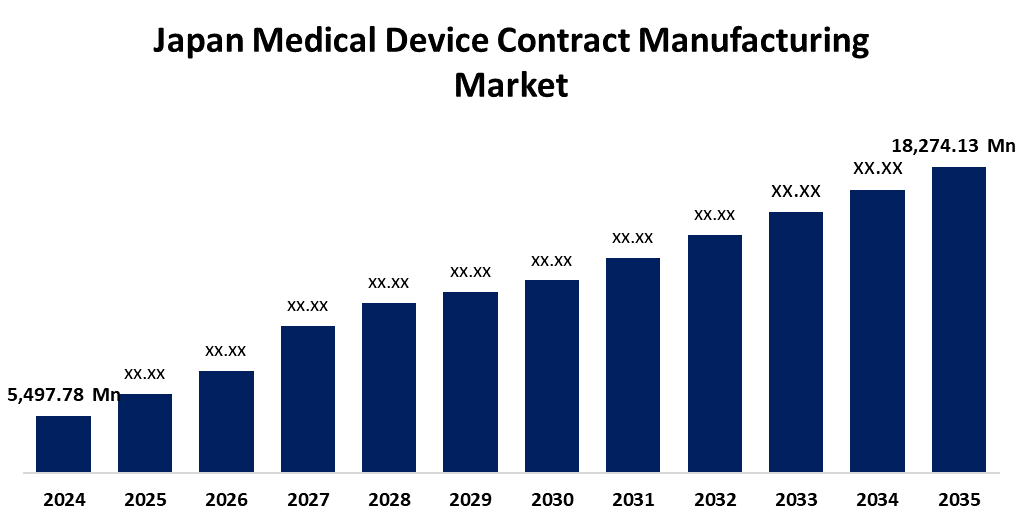
- The Japan Medical Device Contract Manufacturing Market Size was estimated at USD 5,497.78 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 11.54% from 2025 to 2035
- The Japan Medical Device Contract Manufacturing Market Size is Expected to Reach USD 18,274.13 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the Japan Medical device contract manufacturing Market is anticipated to reach USD 18,274.13 million by 2035, growing at a CAGR of 11.54% from 2025 to 2035. Japan's medical device contract manufacturing market is driven by rising demand for advanced medical technologies, an aging population, and increasing healthcare expenditure.
Market Overview
The Japan medical device contract manufacturing market refers to the industry where medical device companies outsource production to specialized manufacturers with technical expertise and regulatory compliance capabilities. Medical devices in Japan are subject to strict regulations set by the Pharmaceuticals and Medical Devices Agency (PMDA), which enforces strict requirements for product performance and safety. When it comes to compliance, documentation, and quality assurance, Japanese contract manufacturers are well-suited to handle this intricate regulatory environment. Their knowledge of PMDA regulations, Good Manufacturing Practices (GMP), and ISO certifications makes them desirable partners for both domestic and foreign medical device manufacturers.
Report Coverage
This research report categorizes the market for the Japan medical device contract manufacturing market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan medical device contract manufacturing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan medical device contract manufacturing market.
Japan Medical Device Contract Manufacturing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 5,497.78 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 11.54% |
| 2035 Value Projection: | USD 18,274.13 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 190 |
| Tables, Charts & Figures: | 113 |
| Segments covered: | By Type, By Source and COVID-19 Impact Analysis |
| Companies covered:: | Terumo Corporation, Fujifilm Medical Systems, Seiko Instruments Inc., Olympus Corporation, Hitachi Medical Corporation, Others. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis. |
Get more details on this report -
Driving Factors
The medical device contract manufacturing industry in Japan is being driven by rising demand for cutting-edge medical technologies, an aging population, and increasing healthcare costs. Japanese medical device firms are encouraged by Japan's strict regulatory standards to partner with specialized manufacturers who possess the technical expertise and compliance skills necessary to meet quality standards. In Japan, contract manufacturing solutions are preferred due to the emphasis on cost-effectiveness and faster time-to-market. Technological advancements such as automation, precision engineering, and additive manufacturing are helping to shorten lead times and enhance production capacities.
Restraining Factors
Navigating the intricate and changing regulatory landscape of Japan is one of the biggest obstacles facing the medical device contract manufacturing industry there. Time-to-market and approval procedures are frequently prolonged due to the Pharmaceuticals and Medical Devices Agency's (PMDA) stringent standards for product safety, efficacy, and quality.
Market Segmentation
The Japan medical device contract manufacturing market share is classified into type and source.
- The class I segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan medical device contract manufacturing market is segmented by type into class I, class II, and class III. Among these, the class I segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to their large volume and comparatively straightforward manufacturing procedures. Class I devices, which include low-risk items like surgical equipment and portable diagnostic tools, consist of a consistent portion of the market.
- The device manufacturing segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan medical device contract manufacturing market is segmented by source into accessories manufacturing, assembly, component manufacturing, device manufacturing, packaging and labelling, and other. Among these, the device manufacturing segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. The demand from OEMs seeking to optimize their operations for full-scale, end-to-end production solutions is shown in the largest segment, device manufacturing.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan medical device contract manufacturing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Terumo Corporation
- Fujifilm Medical Systems
- Seiko Instruments Inc.
- Olympus Corporation
- Hitachi Medical Corporation
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan Medical Device Contract Manufacturing Market based on the below-mentioned segments:
Japan Medical Device Contract Manufacturing Market, By Type
- Class I
- Class II
- Class III
Japan Medical Device Contract Manufacturing Market, By Source
- Accessories Manufacturing
- Assembly Manufacturing
- Component Manufacturing
- Device Manufacturing
- Packaging and Labelling
- Others
Need help to buy this report?