Japan Influenza Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Product (Test Kit and Reagents, Instruments, and Others), By Test Type (Molecular Diagnostic Tests and Traditional Diagnostic Tests), By Type of Flu (Type A Flu, Type B Flu, and Type C Flu), By End User (Hospitals, Diagnostic Laboratories, and Others), and Japan Influenza Diagnostics Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Influenza Diagnostics Market Insights Forecasts to 2035
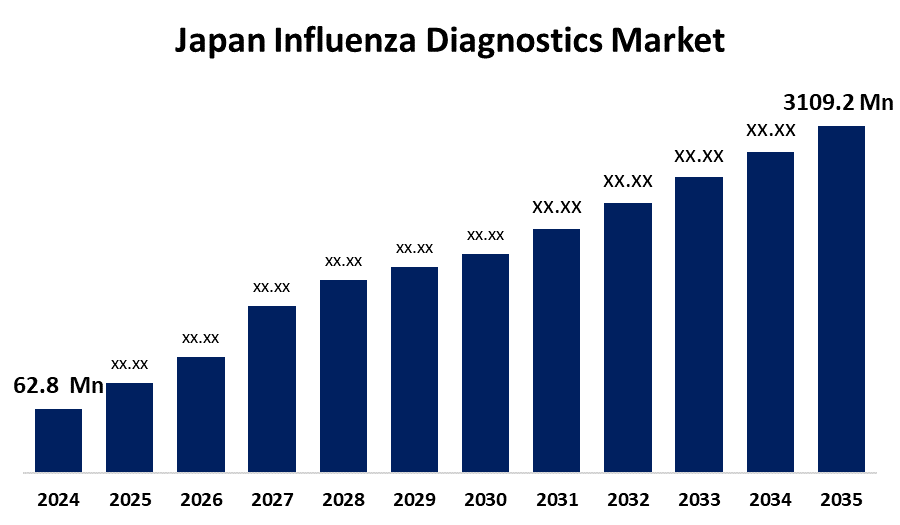
- The Japan Influenza Diagnostics Market Size Was Estimated at USD 62.8 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 5.16% from 2025 to 2035
- The Japan Influenza Diagnostics Market Size is Expected to Reach USD 109.2 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the Japan influenza diagnostics market is anticipated to reach USD 109.2 million by 2035, growing at a CAGR of 5.16% from 2025 to 2035. The Japan market for influenza diagnostics is growing due to its elderly population, increasing cases of influenza outbreaks, and sophisticated diagnostic technologies. The government focus on public health, pandemic preparedness, and early disease detection also drives the growth and uptake of diagnostic solutions.
Market Overview
The Japan influenza diagnostics market refers to the creation and application of tests intended to speedily detect and identify influenza viruses among patients to enable timely treatment and containment. These diagnostics, such as rapid influenza diagnostic tests (RIDTs) and molecular assays, are important for seasonal flu outbreak control and avoidance of complications, particularly among high-risk populations such as the elderly. Market strengths are a developed healthcare infrastructure and a great focus on early detection. There are opportunities to grow rapid diagnostic testing and incorporate advanced molecular diagnostics. Influenza incidence expansion, the aging population of Japan, and technology improvements that improve the accuracy and speed of diagnostics drive market growth. Government action is essential, with initiatives financing diagnostic research and raising public health awareness to improve management of disease. The collaboration, such as the Worldwide Health Innovative Technology Fund (GHIT Fund), facilitates innovation in infectious disease diagnostics.
Report Coverage
This research report categorizes the market for the Japan influenza diagnostics market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan influenza diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan influenza diagnostics market.
Japan Influenza Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 62.8 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 5.16% |
| 2035 Value Projection: | USD 109.2 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 210 |
| Tables, Charts & Figures: | 123 |
| Segments covered: | By Product, By Test Type, By Type of Flu, By End User and COVID-19 Impact Analysis |
| Companies covered:: | Eiken Chemical Co., Ltd., Danaher Corporation, Kyowa Kirin Co., Ltd., Hologic Inc., Sysmex Corporation, Roche Diagnostics, HORIBA, Ltd., Abbott Laboratories, Becton Dickinson and Company, Fujirebio, Quidel Corporation, Thermo Fisher Scientific Inc., Others. |
| Pitfalls & Challenges: | COVID-19 Impact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The Japan influenza diagnostics market is witnessing rising demand, driven by a surge in cases among the elderly and the need for rapid, accurate testing, bolstered by advancements in RIDTs and molecular assay technologies. Government initiatives regarding healthcare infrastructure and financing for the diagnostics of infectious diseases further improve the market growth. Moreover, public health initiatives and increased awareness regarding early diagnosis and treatment of influenza drive the increasing use of sophisticated diagnostic solutions in Japan.
Restraining Factors
The Japan influenza diagnostics market is constrained by high expenditure on sophisticated diagnostics, limited access in remote areas, and complexity in differentiating influenza from other respiratory illnesses. Furthermore, stringent regulatory standards and fluctuating seasonal demand suppress uniform market development.
Market Segmentation
The Japan influenza diagnostics market share is classified into product, test type, type of flu, and end user.
- The test kit and reagents segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan influenza diagnostics market is segmented by product into test kit and reagents, instruments, and others. Among these, the test kit and reagents segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to their ability to detect viral antigens and antibodies in patient specimens, facilitating rapid, accurate diagnosis. New developments improve the sensitivity and specificity of tests, improving flu detection and facilitating on-time medical and public health intervention.
- The traditional diagnostic tests segment dominated the market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan influenza diagnostics market is segmented by test type into molecular diagnostic tests and traditional diagnostic tests. Among these, the traditional diagnostic tests segment dominated the market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is owing to their low cost, ease of use, quick turnaround, and broad availability. These tests are widely applied in clinics and hospitals as a convenient initial flu screen.
- The type A flu segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan influenza diagnostics market is segmented by type of flu into type A flu, type B flu, and type C flu. Among these, the type A flu segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is attributed to its greater rate of transmission, frequent mutation, and relation to seasonal epidemics and pandemics. Its extensive presence leads to increased demand for diagnostic tests, making it the most frequently diagnosed type of flu in Japan.
- The hospitals segment dominated the market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan influenza diagnostics market is segmented by end user into hospitals, diagnostic laboratories, and others. Among these, the hospitals segment dominated the market share in 2024 and is expected to grow at a significant CAGR during the forecast period. Hospitals facilitate prompt access to diagnostic testing for people with flu-like illnesses, enabling timely diagnosis, prompt treatment decisions, and optimum infection control. This is critical to patient health management and preventing the transmission of the virus in healthcare.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan influenza diagnostics market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Eiken Chemical Co., Ltd.
- Danaher Corporation
- Kyowa Kirin Co., Ltd.
- Hologic Inc.
- Sysmex Corporation
- Roche Diagnostics
- HORIBA, Ltd.
- Abbott Laboratories
- Becton Dickinson and Company
- Fujirebio
- Quidel Corporation
- Thermo Fisher Scientific Inc.
- Others
Recent Developments:
- In December 2021, Roche announced plans to launch its SARS-CoV-2 & Flu A/B Rapid Antigen Test for professional use in CE Mark-accepting markets by January. The test enables healthcare professionals to quickly distinguish between COVID-19 and influenza A/B infections. Roche also plans to seek FDA Emergency Use Authorization in early 2022.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan influenza diagnostics market based on the below-mentioned segments:
Japan Influenza Diagnostics Market, By Product
- Test Kit and Reagents
- Instruments
- Others
Japan Influenza Diagnostics Market, By Test Type
- Molecular Diagnostic Tests
- Traditional Diagnostic Tests
Japan Influenza Diagnostics Market, By Type of Flu
- Type A Flu
- Type B Flu
- Type C Flu
Japan Influenza Diagnostics Market, By End User
- Hospitals
- Diagnostic Laboratories
- Others
Need help to buy this report?