Japan Immuno-Oncology Clinical Trials Market Size, Share, and COVID-19 Impact Analysis, By Phase (Phase I, Phase II, Phase III, and Phase IV), By Design (Interventional Trials, Observational Trials, and Expanded Access Trials), By Indication (Solid Tumors and Hematological Cancer), and Japan Immuno-Oncology Clinical Trials Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Immuno-Oncology Clinical Trials Market Insights Forecasts to 2035
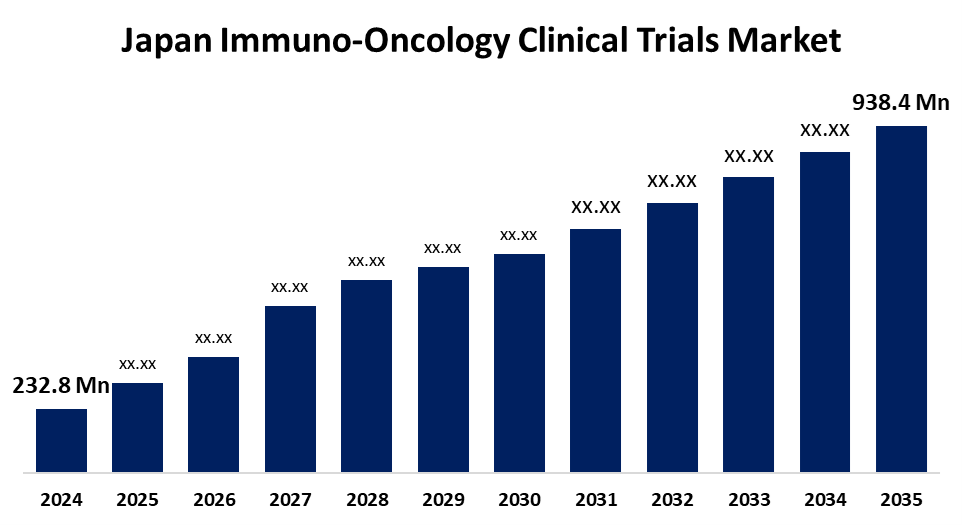
- The Japan Immuno-Oncology Clinical Trials Market Size Was Estimated at USD 232.8 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 13.51% from 2025 to 2035
- The Japan Immuno-Oncology Clinical Trials Market Size is Expected to Reach USD 938.4 Million by 2035
Get more details on this report -
The Japan Immuno-Oncology Clinical Trials Market Size is anticipated to Reach USD 938.4 Million by 2035, Growing at a CAGR of 13.51% from 2025 to 2035. The Japan immuno-oncology clinical trials market is growing with its rising population, robust pharmaceutical sector, and favorable regulatory system. They are stimulating R&D expenditure and increasing the rate of acceptance of immunotherapy as an effective and promising cancer treatment option in the nation.
Market Overview
The Japan Immuno-Oncology Clinical Trials Market Size is defined as advanced therapies like checkpoint inhibitors, cancer vaccines, CAR-T cells, and oncolytic viruses, using trials to harness the bodys immune system to combat cancer more efficiently. Key drivers are Japans aging population, high rates of cancer incidence, robust healthcare infrastructure, robust R&D capacities at research institutes like the Japanese Foundation for Cancer Research, and robust industry-academia collaboration. Technological tools such as genome profiling by SCRUM-Japan and NGS enable accurate patient stratification and biomarker-directed trials. On the horizon are personalized immunotherapies, cancer vaccines, and new modalities like oncolytic viral therapies, supported by clinical trial momentum. The government initiatives like SAKIGAKE, conditional early approval pathways, increased funding, and efforts to rationalize regulation and attract foreign investment are accelerating trial deployment.
Report Coverage
This research report categorizes the market for the Japan immuno-oncology clinical trials market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan immuno-oncology clinical trials market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan immuno-oncology clinical trials market.
Japan Immuno-Oncology Clinical Trials Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 232.8 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 13.51% |
| 2035 Value Projection: | USD 938.4 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 216 |
| Tables, Charts & Figures: | 118 |
| Segments covered: | By Phase, By Indication |
| Companies covered:: | Daiichi Sankyo, Mitsubishi Tanabe Pharma, Kyowa Kirin, Astellas Pharma, Chugai Pharmaceutical, Novartis, Roche, Sumitomo Pharma, Merck & Co., Takeda Pharmaceutical, Pfizer, AstraZeneca, Ono Pharmaceutical, and Other Key Companies. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The Japan immuno-oncology clinical trials market is driven by the high incidence of cancer, aging population, and rising demand for cutting-edge, tailored therapies. Strong government support through schemes such as SAKIGAKE and expedited approval pathways encourages innovation. The advanced healthcare infrastructure of Japan, a healthy R&D environment, and the integration of genomic technologies enable efficient, biomarker-driven trials. Academic-industry-biotech collaborations further enhance clinical development. Additionally, rising investment in precision medicine and immunotherapies propels clinical trial growth across various oncology indications.
Restraining Factors
The Japan immuno-oncology clinical trials market is constrained by high trial and cost complexity, driven by biomarker stratification, prolonged follow-up, and specialized diagnostics. Regulatory rigor, modest patient enrollment, and stiff competition from its neighbours, China and South Korea, add to constrained growth.
Market Segmentation
The Japan immuno-oncology clinical trials market share is classified into phase, design, and indication.
- The phase III segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan immuno-oncology clinical trials market is segmented by phase into phase I, phase II, phase III, and phase IV. Among these, the phase III segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to its pivotal role in determining treatment efficacy, tracking side effects, and contrasting new treatments with current standards. These trials are of greater patient sizes, are of primary importance to regulators, and are heavily sponsored by pharmaceutical companies looking for commercialization.
- The interventional trials segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan immuno-oncology clinical trials market is segmented by design into interventional trials, observational trials, and expanded access trials. Among these, the interventional trials segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to they directly assess the safety and efficacy of new treatments using controlled, systematic means. The trials are the pillar of drug development and approval by control agencies, and attracting significant pharmaceutical investments, and facilitating correct evaluation of immunotherapies in real clinical settings.
- The solid tumors segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan immuno-oncology clinical trials market is segmented by indication into solid tumors and hematological cancer. Among these, the solid tumors segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is attributed to their higher prevalence, including cancer types such as lung, gastric, and colorectal. These cancers are significant unmet clinical needs that have generated extensive research and investment. Immunotherapies for solid tumors have yielded favorable results, triggering increased clinical trials and treatments in this category.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan immuno-oncology clinical trials market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Daiichi Sankyo
- Mitsubishi Tanabe Pharma
- Kyowa Kirin
- Astellas Pharma
- Chugai Pharmaceutical
- Novartis
- Roche
- Sumitomo Pharma
- Merck & Co.
- Takeda Pharmaceutical
- Pfizer
- AstraZeneca
- Ono Pharmaceutical
- Others
Recent Developments:
- In April 2025, Astellas Pharma and the Japanese Foundation for Cancer Research announced a strategic collaboration to accelerate oncology research. Combining Astellas scientific and clinical expertise with JFCRs world-class cancer research capabilities, the partnership aims to advance novel therapies for treatment-resistant cancers more efficiently.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan immuno-oncology clinical trials market based on the below-mentioned segments:
Japan Immuno-Oncology Clinical Trials Market, By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Japan Immuno-Oncology Clinical Trials Market, By Design
- Interventional Trials
- Observational Trials
- Expanded Access Trials
Japan Immuno-Oncology Clinical Trials Market, By Indication
- Solid Tumors
- Hematological Cancer
Need help to buy this report?