Japan Healthcare Contract Research Outsourcing Market Size, Share, and COVID-19 Impact Analysis, By Service (Clinical Trial Services, Clinical Data Management & Biometrics, and Pharmacovigilance), By End-user (Pharmaceutical and Biotech Companies, Medical Devices, and Government Organizations), and Japan Healthcare Contract Research Outsourcing Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Healthcare Contract Research Outsourcing Market Insights Forecasts to 2035
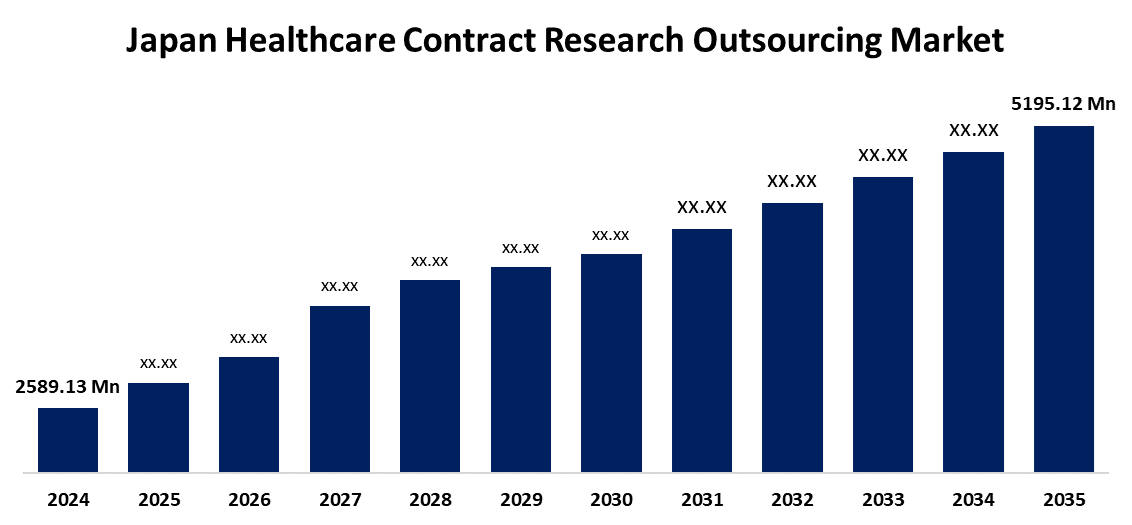
- The Japan Healthcare Contract Research Outsourcing Market Size Was Estimated at USD 2589.13 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 6.54% from 2025 to 2035
- The Japan Healthcare Contract Research Outsourcing Market Size is Expected to Reach USD 5195.12 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the Japan Healthcare Contract Research Outsourcing Market is anticipated to reach USD 5195.12 Million by 2035, growing at a CAGR of 6.54% from 2025 to 2035. Growing investments in clinical research, growing demand for affordable medication development, and government programs to expedite approvals are the main factors propelling the market.
Market Overview
The Japan healthcare contract research outsourcing market focuses on outsourced clinical research services, including clinical trials, regulatory support, and pharmacovigilance, helping pharmaceutical and biotech companies streamline drug development. The need for specialist research services has increased due to the growing complexity of clinical trials, especially in the fields of biologics and precision medicine. CROs are essential in organizing multi-center studies, negotiating Japan's regulatory landscape, and guaranteeing high-quality data collecting. Foreign pharmaceutical companies are increasingly investing in outsourced research services as a result of the growth of international cooperation and Japan's inclusion in the global clinical trial network. With a focus on efficiency, innovation, and regulatory compliance, the market is expected to rise steadily as CROs continue to broaden their service offerings.
Report Coverage
This research report categorizes the market for the Japan healthcare contract research outsourcing market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan healthcare contract research outsourcing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan healthcare contract research outsourcing market.
Japan Healthcare Contract Research Outsourcing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 2589.13 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 6.54% |
| 2035 Value Projection: | USD 5195.12 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Service, By End-user and COVID-19 Impact Analysis |
| Companies covered:: | IQVIA, Labcorp Drug Development, PPD, Covance, Charles River Laboratories, Parexel International, Syneos Health, Celerion and others key players. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
In Japan, the need for outsourced research services is primarily driven by the growing incidence of chronic illnesses and the expanding need for novel treatments. Pharmaceutical and biotechnology firms are using CROs more and more to guarantee regulatory compliance, lower operating expenses, and expedite clinical trial procedures. Further bolstering market expansion are government programs in Japan to expedite drug approval procedures and improve R&D effectiveness. Enhancing trial results and operational efficiency is another benefit of clinical research's quick adoption of cutting-edge technology like artificial intelligence (AI) and big data analytics. Additionally, the quality and scope of clinical trials are being improved by the growing cooperation between academic institutions and CROs.
Restraining Factors
In Japan, the cost of outsourcing research and performing clinical trials is still high because of things like strict quality control procedures, costly site administration, and growing investigator fees. Japan's cost competitiveness issues make it less appealing to small and mid-sized pharmaceutical enterprises looking for reasonably priced research solutions than other Asian markets.
Market Segmentation
The Japan healthcare contract research outsourcing market share is classified into service and end-user.
- The clinical trial services segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan healthcare contract research outsourcing market is segmented by service into clinical trial services, clinical data management & biometrics, and pharmacovigilance. Among these, the clinical trial services segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. The demand for site management procedures, clinical data management, and biometrics services is being driven by the necessity for effective patient recruitment as well as the growing complexity of trial design.
- The pharmaceutical and biotech companies segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan healthcare contract research outsourcing market is segmented by end-user into pharmaceutical and biotech companies, medical devices, and government organizations. Among these, the pharmaceutical and biotech companies segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to their reliance on CROs for efficient and affordable clinical research, pharmaceutical and biotech industries make up the largest end-user category and account for the majority of outsourcing contracts.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan healthcare contract research outsourcing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- IQVIA
- Labcorp Drug Development
- PPD
- Covance
- Charles River Laboratories
- Parexel International
- Syneos Health
- Celerion
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan Healthcare Contract Research Outsourcing Market based on the below-mentioned segments:
Japan Healthcare Contract Research Outsourcing Market, By Service
- Clinical Trial Services
- Clinical Data Management & Biometrics
- Pharmacovigilance
Japan Healthcare Contract Research Outsourcing Market, By End-User
- Pharmaceutical and Biotech Companies
- Medical Devices
- Government Organizations
Need help to buy this report?