Japan Cardiac Safety Services Market Size, Share, and COVID-19 Impact Analysis, By Type (Integrated and Stand-Alone), By Service (ECG/Holter Measurement, Blood Pressure Measurement, Cardiovascular Imaging, Thorough QT Studies, and Others), By End User (Pharmaceutical and Biopharmaceutical Companies, Contract Research Organizations, and Others), and Japan Cardiac Safety Services Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Cardiac Safety Services Market Size Insights Forecasts to 2035
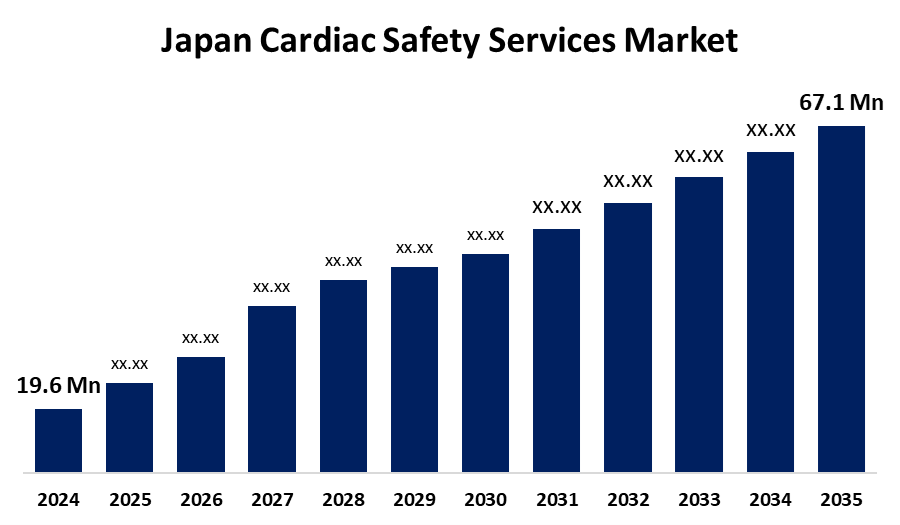
- The Japan Cardiac Safety Services Market Size Was Estimated at USD 19.6 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 11.84% from 2025 to 2035
- The Japan Cardiac Safety Services Market Size is Expected to Reach USD 67.1 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, the Japan cardiac safety services market size is anticipated to reach USD 67.1 million by 2035, growing at a CAGR of 11.84% from 2025 to 2035. The Japan cardiac safety services market is expanding due to increasing cases of cardiovascular disease, an aging population, and advancements in medical technology. Greater patient awareness, favorable reimbursement policies, and greater R&D outlays by healthcare companies contribute further to market growth and demand for services.
Market Overview
The Japan cardiac safety services market refers to ECG/Holter monitoring, blood pressure monitoring, and cardiovascular imaging, is critical for the evaluation of drug, and device generated cardiotoxicity during clinical development and patient management. Strengths are in established surveillance technologies and combining remote monitoring and telehealth into safety protocols. Opportunities arise from growth in clinical trials, particularly decentralized studies, AI-powered telemetry, and blood pressure monitoring services. Growth drivers are Japan aging population and increasing cardiovascular disease burden, driving demand for sophisticated cardiac evaluation and safety services. Government and regulatory authorities, such as the PMDA, favor digital health and enforce strict cardiac safety norms, urging pharma and CROs to invest in thorough cardiac assessments.
Report Coverage
This research report categorizes the market for the Japan cardiac safety services market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan cardiac safety services market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan cardiac safety services market.
Japan Cardiac Safety Services Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 19.6 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 11.84% |
| 2035 Value Projection: | USD 67.1 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 123 |
| Tables, Charts & Figures: | 129 |
| Segments covered: | By Type, By Service, By End User and COVID-19 Impact Analysis |
| Companies covered:: | Nihon Kohden Corporation, Medpace, Agilent Technologies, Terumo Corporation, Boston Scientific, Hitachi Medical Corporation, Clarion Co.,, Nova Research Laboratories, IQVIA, BioClinica, GE Healthcare, Fukuda Denshi Co., Ltd., and Other key vendors |
| Pitfalls & Challenges: | COVID-19 Impact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The Japan cardiac safety services market is driven by a fast-growing population with an increasing incidence of cardiovascular diseases, particularly heart rhythm disorders, which generates high demand for sophisticated monitoring and diagnostic services. Technological advancements, such as ECG/Holter and blood pressure monitoring, and remote or telehealth integration, improve the effectiveness and convenience of cardiac safety testing. Moreover, government initiatives to push digital health, rising investment in healthcare, and favorable PMDA regulatory environments further drive market adoption and service growth.
Restraining Factors
The Japan cardiac safety services market faces challenges due to strict regulatory compliance requirements, high service costs, and the complexities involved in conducting cardiotoxicity assessments. Such requirements call for sophisticated technology and expert specialization, raising barriers to entry and restricting availability in drug development and clinical trials.
Market Segmentation
The Japan cardiac safety services market share is classified into type, service, and end user.
- The integrated segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan cardiac safety services market is segmented by type into integrated and stand-alone. Among these, the integrated segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is due to their integrative nature, aggregating various monitoring tools and analytics. This facilitates clinical workflows, increases efficiency, and aids regulatory compliance, hence highly sought after in clinical trials and hospitals.
- The ECG/holter measurement segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan cardiac safety services market is segmented by service into ECG/holter measurement, blood pressure measurement, cardiovascular imaging, thorough QT studies, and others. Among these, the ECG/holter measurement segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is owing to its broad applicability in real-time heart monitoring, precise detection of arrhythmia, and key position in drug safety assessment, rendering it unavoidable for use in clinical trials and patient diagnosis.
- The pharmaceutical and biopharmaceutical companies segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan cardiac safety services market is segmented by end user into pharmaceutical and biopharmaceutical companies, contract research organizations, and others. Among these, the pharmaceutical and biopharmaceutical companies segment held the largest market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is attributed to their large drug discovery pipelines and stringent regulatory requirements for cardiac safety testing, fuel demand. Advanced cardiac monitoring services are highly dependent upon these companies to assure drug safety, efficacy, and compliance during clinical trials.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan cardiac safety services market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Nihon Kohden Corporation
- Medpace
- Agilent Technologies
- Terumo Corporation
- Boston Scientific
- Hitachi Medical Corporation
- Clarion Co.,
- Nova Research Laboratories
- IQVIA
- BioClinica
- GE Healthcare
- Fukuda Denshi Co., Ltd.
- Others
Recent Developments:
- In November 2023, Meril Life Sciences signed an agreement with Japan Lifeline for the distribution of its Myval Octacor transcatheter heart valve in Japan, subject to PMDA approval. For TAVR/TAVI interventions, Myval Octacor provides a broad range of sizes for greater patient access and a distinct octagonal cell design for better placement accuracy, providing more treatment possibilities than legacy valves.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan cardiac safety services market based on the below-mentioned segments:
Japan Cardiac Safety Services Market, By Type
- Integrated
- Stand-Alone
Japan Cardiac Safety Services Market, By Service
- ECG/Holter Measurement
- Blood Pressure Measurement
- Cardiovascular Imaging
- Thorough QT Studies
- Others
Japan Cardiac Safety Services Market, By End User
- Pharmaceutical and Biopharmaceutical Companies
- Contract Research Organizations
- Others
Need help to buy this report?