Japan Biopharmaceuticals Contract Manufacturing Market Size, Share, and COVID-19 Impact Analysis, By Source (Mammalian and Non-Mammalian), By Service (Process Development, Upstream, Downstream, Fill & Finish Operations, Analytical & QC Studies, Packaging & Labeling, and Others), and Japan Biopharmaceuticals Contract Manufacturing Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareJapan Biopharmaceuticals Contract Manufacturing Market Size Insights Forecasts to 2035
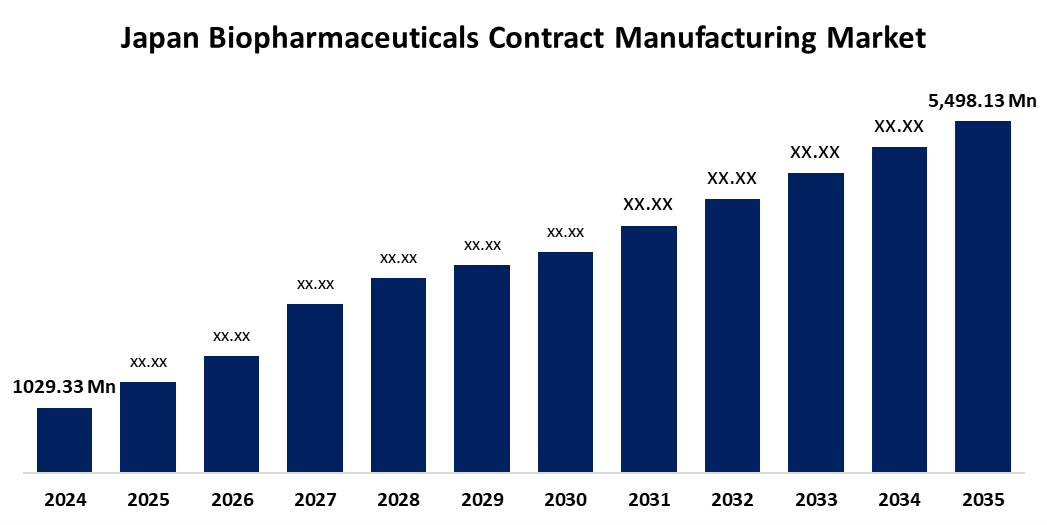
- The Japan Biopharmaceuticals Contract Manufacturing Market Size Was Estimated at USD 1029.33 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 16.45% from 2025 to 2035
- The Japan Biopharmaceuticals Contract Manufacturing Market Size is Expected to Reach USD 5,498.13 Million by 2035
Get more details on this report -
According to a Research Report Published by Spherical Insights & Consulting, the Japan Biopharmaceuticals Contract Manufacturing Market Size is anticipated to reach USD 5,498.13 Million by 2035, growing at a CAGR of 16.45% from 2025 to 2035. The market is driven by Japan’s aging population and rising demand for biologics to treat chronic diseases, alongside the cost-saving benefits of outsourcing. Additionally, advancements in biomanufacturing technologies and the growing presence of global CMOs in Japan are boosting market growth.
Market Overview
The Japan Biopharmaceuticals Contract Manufacturing Market refers to the outsourcing of production processes by biopharmaceutical companies to third-party manufacturers within Japan. In Japan, the need for biologics and biosimilars is being driven by the rising incidence of chronic illnesses such as diabetes, autoimmune disorders, and cancer. Because they provide focused and efficient therapy choices, biopharmaceuticals are becoming more and more popular in the medical field. Pharmaceutical companies are outsourcing production to contract manufacturers more and more because creating and producing these complex chemicals requires sophisticated infrastructure and knowledge.
Report Coverage
This research report categorizes the market for the Japan biopharmaceuticals contract manufacturing market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Japan biopharmaceuticals contract manufacturing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Japan biopharmaceuticals contract manufacturing market.
Japan Biopharmaceuticals Contract Manufacturing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 1029.33 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 16.45% |
| 2035 Value Projection: | USD 5,498.13 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 146 |
| Tables, Charts & Figures: | 116 |
| Segments covered: | By Source, By Service and COVID-19 Impact Analysis |
| Companies covered:: | Boehringer Ingelheim GmbH, Lonza, JRS PHARMA, AGC Biologics, Samsung Biologics, Thermo Fisher Scientific, Inc., ADMA Biologics, Inc., Others and key vendors |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The growing demand for advanced biologics and biosimilars, driven by an aging population and increasing incidence of chronic diseases, is propelling the biopharmaceutical contract manufacturing market in Japan. Contract manufacturing is being used by pharmaceutical businesses to lower production costs, speed up time to market, and concentrate on their core skills. The market is expanding due to the increasing usage of single-use technologies and improvements in cell culture methods that increase production scalability and efficiency. Favorable government initiatives and regulatory assistance for biopharmaceutical research are other important factors. The increasing focus on individualized medicine and the incorporation of digital technologies like automation and data analytics into manufacturing processes are two important trends.
Restraining Factors
The lengthy approval processes and heavy regulatory compliance load in Japan are major barriers that might hinder international collaboration and prolong manufacturing cycles. Scalability and innovation are further hampered by the shortage of qualified workers in the biologics manufacturing industry. Furthermore, it's still difficult to sustain technology transfer and quality control across contract procedures.
Market Segmentation
The Japan biopharmaceuticals contract manufacturing market share is classified into source and service.
- The mammalian segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan biopharmaceuticals contract manufacturing market is segmented by source into mammalian and non-mammalian. Among these, the mammalian segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is because of their capacity to generate complex proteins and biologics with high yields and appropriate post-translational modifications; mammalian systems—more specifically, Chinese Hamster Ovary (CHO) cells dominate the market.
- The process development segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Japan biopharmaceuticals contract manufacturing market is segmented by service into process development, upstream, downstream, fill & finish operations, analytical & qc studies, packaging & labeling, and others. Among these, the process development segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. Process development is still essential as pharmaceutical businesses look to optimize their production processes for scalability and efficiency.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Japan biopharmaceuticals contract manufacturing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Boehringer Ingelheim GmbH
- Lonza
- JRS PHARMA
- AGC Biologics
- Samsung Biologics
- Thermo Fisher Scientific, Inc.
- ADMA Biologics, Inc.
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Japan, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Japan Biopharmaceuticals Contract Manufacturing Market based on the below-mentioned segments:
Japan Biopharmaceuticals Contract Manufacturing Market, By Source
- Mammalian
- Non-Mammalian
Japan Biopharmaceuticals Contract Manufacturing Market, By Service
- Process Development
- Upstream, Downstream
- Fill & Finish Operations
- Analytical & QC Studies
- Packaging & Labeling
- Others
Need help to buy this report?