Germany Hepatitis C Testing Market Size, Share, and COVID-19 Impact Analysis, By Test Type (HCV RNA Tests, HCV Serologic Tests (HCV Ab), HCV Genotype Testing), By End-Users (Diagnostics Labs, Hospitals, Others), and Germany Hepatitis C Testing Market Insights, Industry Trends, Forecasts to 2033
Industry: HealthcareGermany Hepatitis C Testing Market Insights Forecasts to 2033
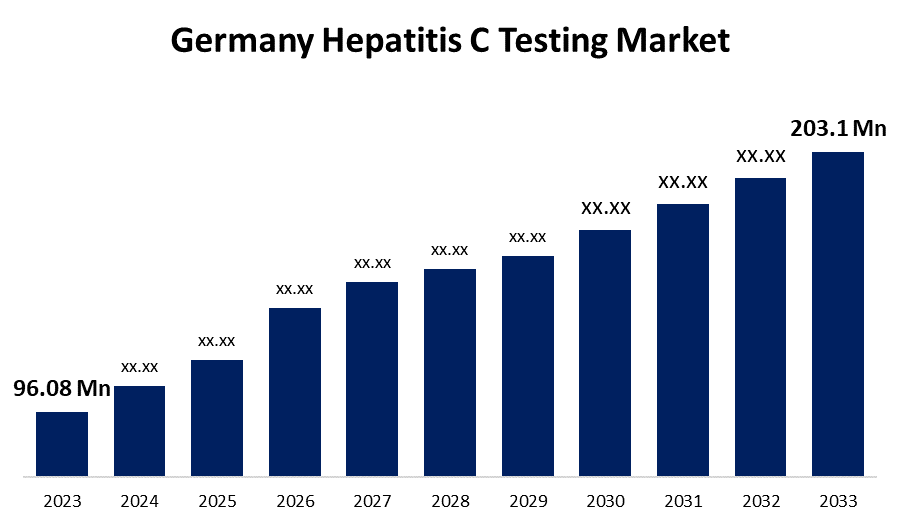
- The Germany Hepatitis C Testing Market Size was valued at USD 96.08 Million in 2023.
- The Market is Growing at a CAGR of 7.7% from 2023 to 2033.
- The Germany Hepatitis C Testing Market Size is expected to reach USD 203.1 Million by 2033.
Get more details on this report -
The Germany Hepatitis C Testing Market Size is anticipated to exceed USD 203.1 Million by 2033, growing at a CAGR of 7.7% from 2023 to 2033.
The growth of Germany's Hepatitis C Testing Market is fueled by the increasing occurrence of hepatitis C, government initiatives, and the influx of migrants in the country. Moreover, the increasing healthcare awareness in Germany is driving the demand for the hepatitis C testing market.
Market Overview
Hepatitis C testing refers to the procedure for screening individuals for the presence of the hepatitis C virus (HCV) in their blood. Hepatitis C is a viral infection that primarily affects the liver and can lead to chronic liver disease, cirrhosis, and liver cancer if left untreated. The testing is typically done through blood tests which detect the presence of HCV antibodies or HCV RNA. Hepatitis C is one of the highest prevalent infections in Germany. Multiple initiatives have been launched throughout the country to further investigate illness patterns, including occurrence and the most common genotypes. Hepatitis C is transmitted by contact with infected blood or the sharing of needles or other drug-preparation and injection equipment. The most effective way to prevent hepatitis C is by excluding practices that can spread the disease, such as drug injection and getting tested for hepatitis. Furthermore, the country's expanding migrant population is predicted to raise the occurrence of HCV infections, driving the need for Germany Hepatitis C Testing.
Report Coverage
This research report categorizes the market for the Germany hepatitis c testing market based on various segments and regions forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the hepatitis c testing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the hepatitis c testing market.
Germany Hepatitis C Testing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 96.08 Million |
| Forecast Period: | 2023-2033 |
| Forecast Period CAGR 2023-2033 : | 7.7% |
| 2033 Value Projection: | USD 203.1 Million |
| Historical Data for: | 2019-2022 |
| No. of Pages: | 178 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Test Type, By End-Users and COVID-19 Impact Analysis. |
| Companies covered:: | Abbott, Bio-Rad Laboratories, Inc., DiaSorin S.p.A., Roche Diagnostics, Siemens Healthcare, DiaSorin S.P.A, AbbVie, GlaxoSmithKline, Ever Pharma, Alpen Pharma Group, Amgen and other key vendors. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Factors such as improper injection techniques, blood transfusions, and intravenous drug usage all help to propagate the infection. The general public and physicians are becoming more aware of the advantages of early diagnosis, resulting in higher testing rates. The increased incidence of Hepatitis C infections in Germany is a significant driver of the testing market's expansion. The WHO's goal is consistent with Germany's HCV elimination strategy (BIS 2030), which is an integrated approach to HIV, hepatitis B, C, and other STDs. This strategy aims to improve German public health by preventing diseases such as AIDS, liver cirrhosis, and HCC. Germany has two types of health insurance such as legal and commercial. Patients are not involved in financial decisions, and copayments are reduced to a minimum in legal health. There is an agreement between the sick fund and service providers under legal health. HCV testing and treatment are covered by the legal health insurance plan. All German nationals have access to the healthcare system through legal health insurance, and they can choose to opt out of commercial health insurance. The above-mentioned government attempt is projected to help boost the market development for hepatitis C testing in the next years.
Restraining Factors
One of the most significant impediments to the widespread acceptance of hepatitis C diagnosis in Germany is the lack of a comprehensive testing program. Hepatitis C testing has been implemented nationwide despite an increase in disease incidence due to a scarcity of a broad HCV testing strategy. Numerous clinical articles indicate that there is a definite requirement in Germany for a national hepatitis C testing approach. Furthermore, the expense of hepatitis c testing procedures, particularly modern molecular assays, can be a considerable limitation to testing for some population groups.
Market Segmentation
The Germany hepatitis c testing market share is classified into test type and end-users.
- The serologic tests (HCV Ab) segment is expected to hold a significant share of the Germany hepatitis c testing market during the forecast period.
The Germany hepatitis c testing market is segmented by test type into HCV RNA tests, HCV serologic tests (HCV Ab), and HCV genotype testing. Among these, the serologic tests (HCV Ab) segment is expected to hold a significant share of the Germany hepatitis c testing market during the forecast period. The need for hepatitis C testing in Germany is expected to rise due to a critical need for monitoring vulnerable and general populations for HCV antibodies. This blood test determines the body's antibodies for fighting the infection. When the body identifies the hepatitis C virus in the blood, it synthesizes these proteins.
- The hospitals segment is expected to hold the largest share of the Germany hepatitis c testing market during the forecast period.
Based on the end-users, the Germany hepatitis c testing market is divided into diagnostics labs, hospitals, and others. Among these, the hospitals segment is expected to hold the largest share of the Germany hepatitis c testing market during the forecast period. The predominant position of this segment is because the German healthcare system includes a significant number of medical centers and hospitals that regularly prescribe and test for hepatitis C.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Germany hepatitis c testing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott
- Bio-Rad Laboratories, Inc.
- DiaSorin S.p.A.
- Roche Diagnostics
- Siemens Healthcare
- DiaSorin S.P.A
- AbbVie
- GlaxoSmithKline
- Ever Pharma
- Alpen Pharma Group
- Amgen
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Recent Development
- In July 2023, the Centers for Illness Control and Prevention (CDC) issued new guidance proposing that clinicians use RNA diagnosis on all hepatitis C virus (HCV) antibody reactive samples to improve the rate and efficiency of viral illness testing.
Market Segment
This study forecasts country revenue from 2020 to 2033. Spherical Insights has segmented the Germany washing machine market based on the below-mentioned segments:
Germany Hepatitis C Testing Market, By Test Type
- HCV RNA Tests
- HCV Serologic Tests (HCV Ab)
- HCV Genotype Testing
Germany Hepatitis C Testing Market, By End-Users
- Diagnostics labs
- Hospitals
- Others
Need help to buy this report?