France Rare Disease Genetic Testing Market Size, Share, And COVID-19 Impact Analysis, By Disease (Neurological Disorders, Immunological Disorders, Hematology Diseases, Endocrine & Metabolism Diseases, Musculoskeletal Disorders, Cardiovascular Disorders, Dermatology Diseases, and Other Rare Diseases), By Specialty (Molecular Genetic Tests, Chromosomal Genetic Tests, Biochemical Genetic Tests) By End User (Research Laboratories & CROs, Hospitals & Clinics, and Diagnostic Laboratories), and France Rare disease genetic testing Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareFrance Rare Disease Genetic Testing Market Insights Forecasts to 2033
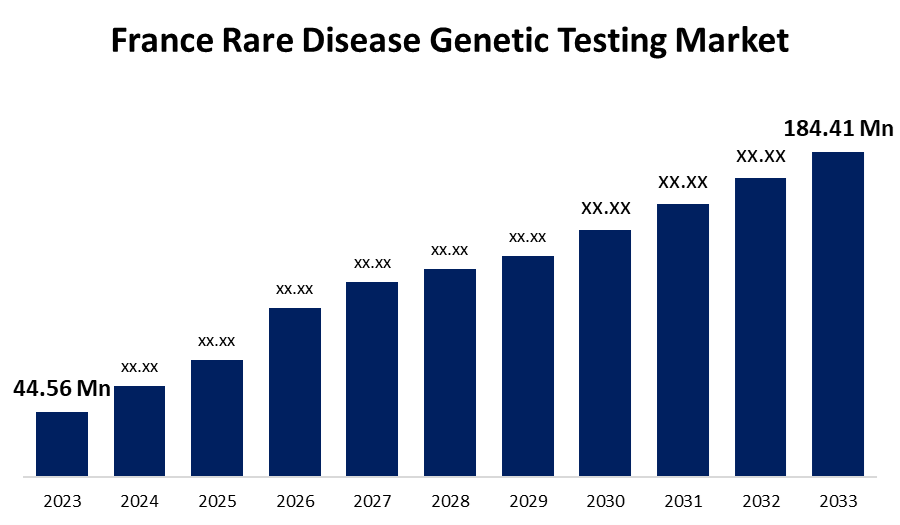
- The France Rare Disease Genetic Testing Market Size was valued at USD 44.56 Million in 2023.
- The France Rare Disease Genetic Testing Market Size is Expected to Grow at a CAGR of around 15.26% from 2023 to 2033.
- The France Rare Disease Genetic Testing Market Size is Expected to Reach USD 184.41 Million by 2033.
Get more details on this report -
The France Rare Disease Genetic Testing Market size is predicted to Grow from USD 44.56 Million in 2023 to USD 184.41 Million by 2033 at a CAGR of 15.26% during the forecast period. The market Growth is driven by government support, technology breakthroughs in the healthcare sector, and Growing health consciousness among local consumers.
Market Overview
The French rare disease genetic testing market refers to the business that supports the healthcare sector, which emphasizes the medical procedures that detect genetic changes or abnormalities associated with rare diseases. These tests examine proteins, chromosomes, or genes to determine therapy options, evaluate risks, or validate suspected disorders. Technologies include chromosomal analysis, PCR-based testing, and next-generation sequencing (NGS) are supported by this healthcare subsidiary. Hospitals, diagnostic facilities, and research labs are among the industries it supports. Market growth is driven by several opportunities such as the healthcare is evolving as a consequence of the increasing acceptance of non-invasive genetic testing techniques like blood- and saliva-based assays This is because these tests are fewer invasive and easier to perform, more companies might get inspired to create and market diagnostics specifically designed for rare disease diagnosis. Further, these non-invasive alternatives have a lot of promise for businesses trying to break new ground in the genetic testing industry.
Report Coverage
This research report categorizes the France rare disease genetic testing market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyzes the key growth drivers, opportunities, and challenges influencing the France rare disease genetic testing market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyzes their core competencies in each sub-segment of the France rare disease genetic testing market.
France Rare Disease Genetic Testing Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 44.56 Million |
| Forecast Period: | 2023 - 2033 |
| Forecast Period CAGR 2023 - 2033 : | 15.26% |
| 2033 Value Projection: | USD 184.41 Million |
| Historical Data for: | 2019 - 2022 |
| No. of Pages: | 230 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Disease, By Specialty, By End User |
| Companies covered:: | Sanofi, Eurofins Scientific, PathoQuest, Erytech Pharma, NeurATRIS, and Others |
| Pitfalls & Challenges: | COVID-19 Empact,Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The rare disease genetic testing market in France has been expanding significantly due to the increasing prevalence of these diseases, emphasizing the urgent need for innovative techniques for diagnosis. Further, the market growth is driven by an upsurge in sophisticated supplies made especially for detecting rare illnesses, which provides additional support for the expansion of this industry. Moreover, growing awareness of uncommon genetic illnesses, the need for tailored therapy, and developments in next-generation sequencing (NGS) are driving this market expansion. Further, driving market expansion is supported by government programs and financing for rare illness research, as well as easier access to genetic counseling. Besides, partnerships between research institutes and biotech companies improve diagnostic skills, increasing market need, and their expansion.
Restraints & Challenges
The French rare disease genetic testing market growth is being hampered by several obstacles, such as less awareness among the local consumer for such rare diseases and treatment methodology. Moreover, sometimes a delayed permission process from regulatory authorities hammers the market growth.
Market Segmentation
The France rare disease genetic testing market share is classified into disease, specialty, and end user.
- The immunological disorders segment accounted for the largest share of the France rare disease genetic testing market in 2023 and is expected to grow at a significant CAGR over the forecast period.
Based on disease, the France rare disease genetic testing market is categorized into neurological disorders, immunological disorders, hematology diseases, endocrine & metabolism diseases, musculoskeletal disorders, cardiovascular disorders, dermatology diseases, and other rare diseases.Among these, the immunological disorders segment accounted for the largest share of the France rare disease genetic testing market in 2023 and is expected to grow at a significant CAGR over the forecast period. This is because of the increasing need for prompt and precise diagnosis due to growing immunologic rare illnesses, such as autoimmunity, autoinflammatory syndromes, and primary immune system disorders (PIDs). Moreover, genetic insights are becoming essential in addressing diseases with the additional support of rising awareness and newborn screening initiatives. Further, research in genetic diagnostics is rapidly changing due to government programs and regulatory backing, with the development of targeted treatments and personalized medicine.
- The molecular genetic tests segment accounted for the largest share of the France rare disease genetic testing market in 2023 and is projected to grow at a remarkable CAGR during the forecast period.
Based on the specialty, the France rare disease genetic testing market is categorized into molecular genetic tests, chromosomal genetic tests, and biochemical genetic tests. Among these, the molecular genetic tests segment accounted for the largest share of the France rare disease genetic testing market in 2023 and is projected to grow at a remarkable CAGR during the forecast period. This is due to rapid results of technology advancements and the substantial investment in healthcare facilities, such as novel methods for DNA sequencing, comprehensive genetic testing, and facilitating precision medicine, which have improved patient outcomes. Beyond that, segment growth is further accelerated by the major market players' development of their genetic testing portfolios as well as rising investments in precision diagnostics and customized medicine.
- The research laboratories & CROs segment accounted for the largest share of the France rare disease genetic testing market in 2023 and is projected to grow at a substantial CAGR during the forecast period.
Based on the mode of end user, the France rare disease genetic testing market is segmented into research laboratories & CROs, hospitals & clinics, and diagnostic laboratories. Among these, the research laboratories & CROs segment accounted for the largest share of the France rare disease genetic testing market in 2023 and is projected to grow at a substantial CAGR during the forecast period. This segment growth is attributed to the market expansion because of considerable blood samples are sent to a lab for analysis, and modern testing technology makes it easier, based on many specialties, such as chromosomal, biochemical, and molecular genetic tests. Additionally, labs provide genetic testing, including those certified by CLIA for clinical cytogenetics, pathology, and chemistry, among other disciplines, which highlights the proficiency, transparency might be addressed to healthy volunteers for such engagement.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the France rare disease genetic testing market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Sanofi
- Eurofins Scientific
- PathoQuest
- Erytech Pharma
- NeurATRIS
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Key Market Developments
- In April 2025, the France Genomic Medicine Initiative, also known as Plan France Medecine Genomique 2025 (PFMG 2025), was a transformative program aimed at integrating genomic medicine into routine healthcare. It focused on personalized diagnostics and treatments through genome sequencing, initially targeting rare diseases and cancers, and eventually expanding to common diseases like cardiovascular and neurological disorders. The initiative also emphasized technological innovation, economic growth, and equitable access to genomic medicine across France.
- In February 2025, France's fourth national rare diseases plan (PNMR4) was a significant initiative to improve care for individuals with rare diseases. It focused on three main pillars: care, research, and industry. The plan included expanding specialized care centers, enhancing genetic testing and genomic sequencing for early diagnosis, and boosting research to develop new treatments.
Market Segment
This study forecasts revenue at France, regional, and country levels from 2023 to 2033. Spherical Insights has segmented the France rare disease genetic testing market based on the below-mentioned segments:
France Rare Disease Genetic Testing Market, By Disease
- Neurological Disorders
- Immunological Disorders
- Hematology Diseases
- Endocrine & Metabolism Diseases
- Musculoskeletal Disorders
- Cardiovascular Disorders
- Dermatology Diseases
- Other Rare Diseases
France Rare Disease Genetic Testing Market, By Specialty
- Molecular Genetic Tests
- Chromosomal Genetic Tests
- Biochemical Genetic Tests
France Rare Disease Genetic Testing Market, By End User
- Research Laboratories & CROs
- Hospitals & Clinics
- Diagnostic Laboratories
Need help to buy this report?