France Rare Disease Clinical Trials Market Size, Share, And COVID-19 Impact Analysis, By Phases (Phase I, Phase II, Phase III, Phase IV), By Therapeutic Areas (Oncology, Cardiovascular Disorders, Neurological Disorders, Infectious Disease, Genetic Disorders, Autoimmune and Inflammation, Hematologic Disorders, Musculoskeletal Disorders, and Others), and France Rare Disease Clinical Trials Market Insights, Industry Trend, Forecasts to 2033
Industry: HealthcareFrance Rare Disease Clinical Trials Market Insights Forecasts To 2033
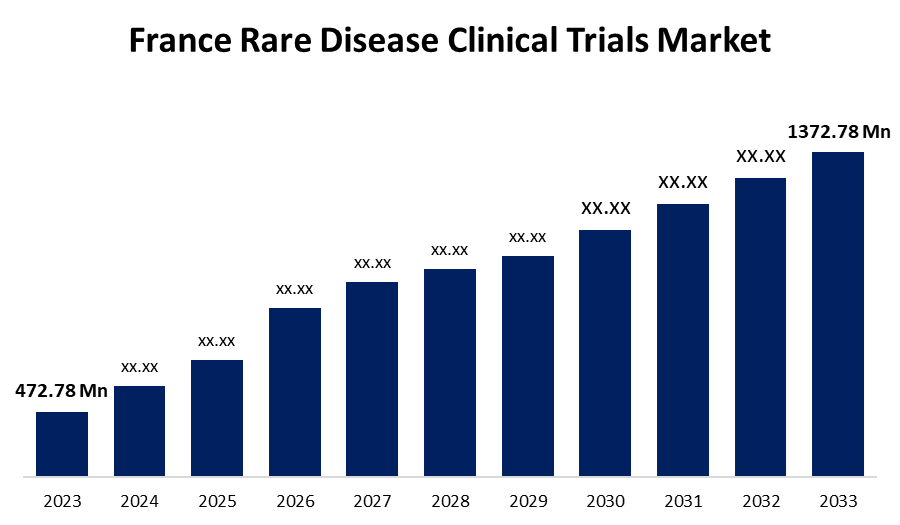
- The France Rare Disease Clinical Trials Market Size was valued at USD 472.78 Million in 2023.
- The France Rare Disease Clinical Trials Market Size is Expected to Grow at a CAGR of around 12.12% from 2023 to 2033.
- The France Rare Disease Clinical Trials Market Size is Expected to Reach USD 1372.78 Million by 2033.
Get more details on this report -
The France Rare Disease Clinical Trials Market size is predicted to Grow from USD 472.78 Million in 2023 to USD 1372.78 Million by 2033 at a CAGR of 11.25% during the forecast period. The market growth is driven by strong government support and growing awareness among the locals about rare disease and healthy welfare resource.
Market Overview
The French rare disease clinical trials market refers to the industry that supports the healthcare sector by introducing a clinical trial in different phases for rare diseases to reduce harmful outcomes and detect a causative factor before it becomes a tragedy. Further, these trials are conducted with a written description of any therapeutic or diagnostic agent conducted in human subjects, in which the clinical and statistical description, presentations, and analysis are fully integrated into a single report. The efficiency of drug discovery and trials is anticipated to be improved by the growing use of artificial intelligence and biomarker-driven research. Moreover, patient engagement and data collection are enhanced via decentralized clinical trials, which are bolstered by digital health tools and remote monitoring. Beyond that, the key market players with their collaborative projects boost the country's healthcare industry, such as in May 2024, Pfizer and AstraZeneca announced plans to invest nearly dollor 1 billion in France, showcasing their commitment to expanding operations in the country. Pfizer intended to allocate €500 million to enhance its research and development capabilities, while AstraZeneca planned to invest $388 million to strengthen its existing facilities, particularly its Dunkirk site, which focused on producing inhaled devices.
Report Coverage
This research report categorizes the France rare disease clinical trials market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyzes the key growth drivers, opportunities, and challenges influencing the France rare disease clinical trials market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyzes their core competencies in each sub-segment of the France rare disease clinical trials market.
France Rare Disease Clinical Trials Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2023 |
| Market Size in 2023: | USD 472.78 Million |
| Forecast Period: | 2023 - 2033 |
| Forecast Period CAGR 2023 - 2033 : | 11.25% |
| 2033 Value Projection: | USD 1372.78Million |
| Historical Data for: | 2019 - 2022 |
| No. of Pages: | 230 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Phases, By Therapeutic Areas |
| Companies covered:: | AbbVie Inc., Alexion Pharmaceuticals, Inc., Amgen Inc, AstraZeneca PLC, Baxter International, Bayer AG, Biogen, Bristol-Myers Squibb, Eli Lilly and Company, GSK plc, Johnson & Johnson, Merck & Co. Inc., Novartis AG, Novo Nordisk, Pfizer, Inc., Pharmacyclics LLC, Sanofi SA, Seagen Inc., Takeda Pharmaceutical Company Limited., Vertex Pharmaceutical, and Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The rare disease clinical trials market in France has been expanding significantly due to the increasing prevalence of rare disease cases and the rising need for innovative techniques during the diagnosis process. Further, the market growth is driven by sophisticated supplies of techniques made for detecting rare illnesses, which provides additional support from key market players in this industry. Moreover, rising awareness of rare genetic illnesses and their related tailored therapy is driving this market expansion. Further, driving market expansion is supported by government programs and financing for rare illness research, as well as easier access to genetic counseling. Besides, partnerships between research institutes and biotech companies improve diagnostic skills, increasing market need, and their expansion.
Restraints & Challenges
The French rare disease clinical trials market expansion is being constrained by some barriers, such as less awareness among the local citizens of such rare diseases and treatment methodology. Moreover, a delayed permission during the enrollment process from regulatory authorities reduces the market growth.
Market Segmentation
The France rare disease clinical trials market share is classified into phases and therapeutic areas.
- The phase III segment accounted for the largest share of the France rare disease clinical trials market in 2023 and is expected to grow at a significant CAGR over the forecast period.
Based on phase, the France rare disease clinical trials market is categorized into phase I, phase II, phase III, and phase IV. Among these, the phase III segment accounted for the largest share of the France rare disease clinical trials market in 2023 and is expected to grow at a significant CAGR over the forecast period. This is because phase III trials are frequently necessary to evaluate the long-term safety and effectiveness of complex rare illness treatments. Further, collaborating with specialist research facilities and stepping up an increasing number of late-stage studies might also be attributed to regulatory incentives that support research on rare diseases. This segment's dominance in the rare illness clinical trials market has been strengthened by the need for strong clinical evidence to support regulatory approvals.
- The oncology segment accounted for the highest share of the France rare disease clinical trials market in 2023 and is projected to grow at a substantial CAGR during the forecast period.
Based on the therapeutic area, the France rare disease clinical trials market is categorized into oncology, cardiovascular disorders, neurological disorders, infectious disease, genetic disorders, autoimmune and inflammation, hematologic disorders, musculoskeletal disorders, and others. Among these, the oncology segment accounted for the highest share of the France rare disease clinical trials market in 2023 and is projected to grow at a substantial CAGR during the forecast period. This is due to clinical trials for rare cancers have risen drastically as a result of the rise of targeted treatments and immunotherapies. Furthermore, the ability to identify rare cancer subtypes precisely has been made possible by advancements in genomic profiling, which has supported more targeted medication development. Moreover, segment has increased significantly due to the growing interest in innovative treatments, and accelerated trial enrollment is a result of increased cooperation between pharmaceutical corporations and research organizations.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the France rare disease clinical trials market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- AbbVie Inc.
- Alexion Pharmaceuticals, Inc.
- Amgen Inc
- AstraZeneca PLC
- Baxter International
- Bayer AG
- Biogen
- Bristol-Myers Squibb
- Eli Lilly and Company
- GSK plc
- Johnson & Johnson
- Merck & Co. Inc.
- Novartis AG
- Novo Nordisk
- Pfizer, Inc.
- Pharmacyclics LLC
- Sanofi SA
- Seagen Inc.
- Takeda Pharmaceutical Company Limited.
- Vertex Pharmaceutical
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Key Market Developments
- In March 2025, Medable achieved a significant milestone by securing regulatory approval from France's CNIL (Commission Nationale de l’Informatique et des Libertés). This breakthrough was particularly impactful for global clinical trials, as it set a precedent for future operations in France and beyond. The approval was achieved in collaboration with Servier, a major French pharmaceutical sponsor, and covered two global clinical studies in phases two and three.
- In September 2024, the European Rare Diseases Research Alliance (ERDERA) marked a transformative moment in rare disease research. With an estimated budget of 380 million, ERDERA aims to improve the lives of 30 million rare disease patients across Europe and beyond. ERDERA, under the leadership of INSERM (France), united over 170 organizations spanning the public and private sectors. This groundbreaking alliance aimed to propel research efforts in rare disease prevention, diagnosis, and treatment, leveraging insights and resources established by earlier initiatives, such as the European Joint Programme on Rare Diseases (EJP RD).
Market Segment
This study forecasts revenue at France, regional, and country levels from 2023 to 2033. Spherical Insights has segmented the France rare disease clinical trials market based on the below-mentioned segments:
France Rare Disease Clinical Trials Market, By Phases
- Phase I
- Phase II
- Phase III
- Phase IV
France Rare Disease Clinical Trials Market, By Therapeutic Areas
- Oncology
- Cardiovascular Disorders
- Neurological Disorders
- Infectious Disease
- Genetic Disorders
- Autoimmune and Inflammation
- Hematologic Disorders
- Musculoskeletal Disorders
- Others
Need help to buy this report?