France Prostate Cancer Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Preliminary Tests, Confirmatory Tests), By Type (Adenocarcinoma, Interstitial Cell Carcinoma, Other), and Prostate Cancer Diagnostics Market Insights, Industry Trend, Forecasts to 2035.
Industry: HealthcareFrance Prostate Cancer Diagnostics Market Insights Forecasts to 2035
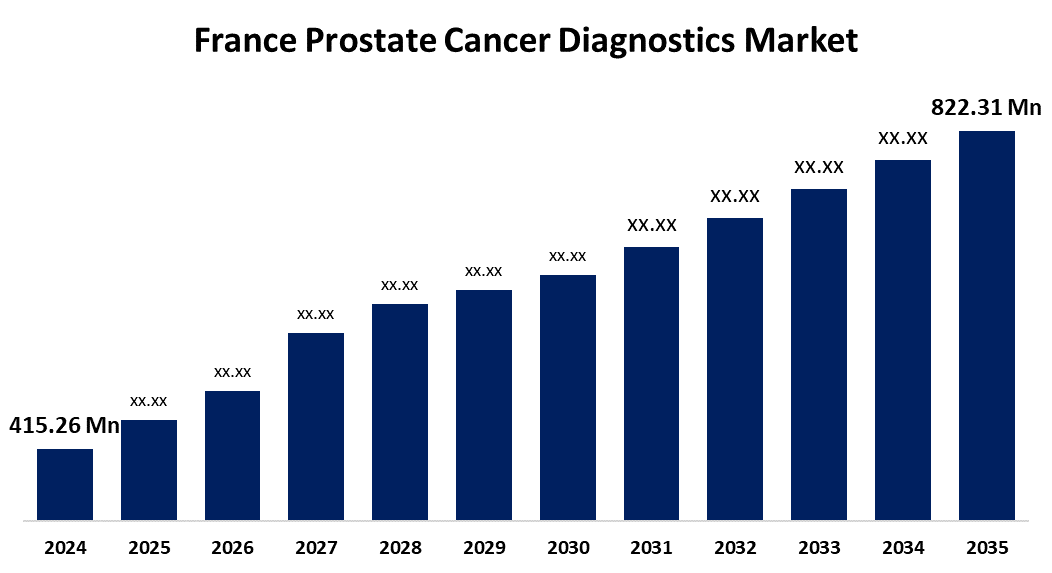
- The France Prostate cancer diagnostics market Size was estimated at USD 415.26 Million in 2024
- The France Market Size is Expected to Grow at a CAGR of around 6.41% from 2025 to 2035
- The France Prostate cancer diagnostics market Size is Expected to Reach USD 822.31 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, The France Prostate Cancer Diagnostics Market Size is Anticipated to reach USD 822.31 Million By 2035, Growing at a CAGR of 6.41% from 2025 to 2035. The strong support from the government and growing changes in lifestyle, and collaborative projects of key companies boost the market growth.
Market Overview
The france prostate cancer diagnostics market is a subpart of the healthcare industry, through the introduction of businesses that focus on prostate cancer diagnostics methods are developed and distributed by this sector to detect prostate cancer. The majority of the tests used in this industry are preliminary and confirmatory assays. The purpose of these tests is to identify several forms of prostate cancer, including interstitial cell carcinoma, adenocarcinoma, and others. Hospitals, home care agencies, outpatient clinics, research and manufacturing, and a few others are among the industry's many consumers. Also, market expansion is mostly driven by technological improvements. The use of cutting-edge technologies like liquid biopsies, AI in diagnostic imaging, and more precise PSA tests is enhancing the early diagnosis of cancer. These developments draw patients and healthcare professionals with improved diagnostic precision and non-invasive substitutes. Concurrently, development news, like in February 2024, the International Agency for Research on Cancer (IARC) hosted the PRAISE-U consortium meeting in Lyon, france. The PRAISE-U project aimed to reduce morbidity and mortality caused by prostate cancer in the European Union by providing evidence for a risk-stratified approach to early detection.
Report Coverage
This research report categorizes the market for the france prostate cancer diagnostics market based on various segments and regions and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the france prostate cancer diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the france prostate cancer diagnostics market.
France Prostate Cancer Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 415.26 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 6.41% |
| 2035 Value Projection: | USD 822.31 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 190 |
| Tables, Charts & Figures: | 90 |
| Segments covered: | By Test Type, By Type and COVID-19 Impact Analysis |
| Companies covered:: | OncoDiag, OWKIN, Phost’in Therapeutics, Pixience, Brenus Pharma, Abbott Laboratories, Siemens Healthineers AG, and other key vendors. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The france market for prostate cancer diagnostics is primarily driven by the rising incidence of prostate cancer, which is caused by several reasons such as an aging population, changing lifestyles, and increased awareness and screening initiatives. This entails setting up screening programs, expanding access to diagnostic centers, educating medical personnel, and raising awareness of the significance of prostate cancer early detection, which propels the market growth. Additionally, the market for prostate cancer diagnostics is growing due to the presence of multiple local market players who are consistently working to develop high-quality tools while pursuing different approaches like partnerships, acquisitions, introductions, and company growth.
Restraining Factors
The high expense of detecting and treating prostate cancer tools is impeding the company's expansion. Furthermore, the policies of private insurance companies, reimbursement coverage also differ, which hammers the market expansion.
Market Segmentation
The France prostate cancer diagnostics market share is classified into test type and type.
- The confirmatory tests segment held the highest share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The france prostate cancer diagnostics market is differentiated by test type into preliminary tests, and confirmatory tests. Among these, the confirmatory tests segment held the highest share in 2024 and is expected to grow at a significant CAGR during the forecast period. This segment is driven by rising cases of prostate cancer and their effects spreading to other organs, such as the genital and urinary tract. Besides, to determine the risk of metastasis, imaging tests are conducted when the cancer has spread to other organs due to the development of the healthcare sector specific to imaging technology, which is utilized for this test. Confirmatory procedures such as the PCA3 test, transrectal ultrasonography, and biopsy are necessary to confirm the diagnosis if these initial findings are incorrect, which is expected to enhance market growth.
- The adenocarcinoma segment accounted for the highest share in 2024 and is expected to grow at a substantial CAGR during the forecast period.
The france prostate cancer diagnostics market is classified by type into adenocarcinoma, interstitial cell carcinoma, and other. Among these, the adenocarcinoma segment accounted for the highest share in 2024 and is expected to grow at a substantial CAGR during the forecast period. This is because adenocarcinoma is the most common kind of prostate cancer compared to others. Further, prostate adenocarcinoma usually manifests as poor urine flow, difficulties emptying the bladder, trouble urinating, occasional blood in the urine, and chronic lower back pain; such symptoms help to address it easily. Further, as a result of its high development and demand for effective screening and treatment methods, adenocarcinoma is the primary focus of diagnostic and commercial efforts.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the france prostate cancer diagnostics market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- OncoDiag
- OWKIN
- Phost'in Therapeutics
- Pixience
- Brenus Pharma
- Abbott Laboratories
- Siemens Healthineers AG
- Others
Recent Developments:
- In April 2025, Telix Pharmaceuticals' Illuccix PSMA-PET imaging agent officially approved in france. This imaging agent was used for detecting and localizing prostate-specific membrane antigen (PSMA)-positive lesions in adults with prostate cancer. The approval by ANSM (france’s National Agency for Medicines and Health Products Safety) allowed hospitals and clinics to prepare PSMA-PET scans on-site, making diagnosis and treatment decisions more efficient.
- In May 2024, Curium officially launched the first commercial doses of Pylclari in france. Pylclari is an innovative 18F-PSMA PET tracer designed to detect prostate-specific membrane antigen (PSMA)-positive lesions in patients with prostate cancer. In france, the trace is produced at Curium’s facilities in Bordeaux, Lyon, Nancy, Marseille, Paris, and Tours.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at france, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the prostate cancer diagnostics market based on the below-mentioned segments
France Prostate Cancer Diagnostics Market, By Test Type
- Preliminary Tests
- Confirmatory Tests
France Prostate Cancer Diagnostics Market, By Type
- Adenocarcinoma
- Interstitial Cell Carcinoma
- Other
Need help to buy this report?