France Pharmacovigilance Market Size, Share, and COVID-19 Impact Analysis, By Product Life Cycle (Pre-clinical, Phase I, Phase II, Phase III, and Phase IV), By Service Provider (In-house and Contract Outsourcing), and France Pharmacovigilance Market Insights, Industry Trend, Forecasts to 2035.
Industry: HealthcareFrance Pharmacovigilance Market Insights Forecasts to 2035
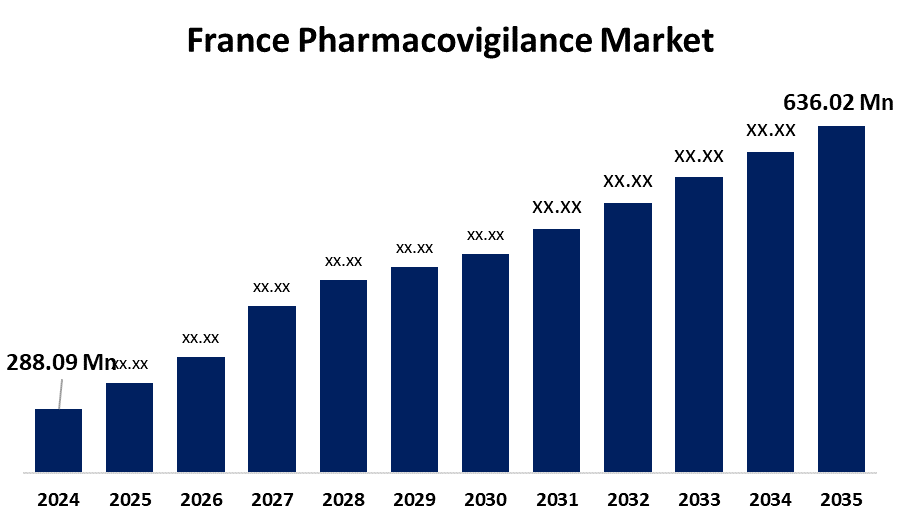
- The France Pharmacovigilance Market Size was estimated at USD 288.09 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 7.47% from 2025 to 2035
- The France Pharmacovigilance Market Size is Expected to Reach USD 636.02 Million by 2035
Get more details on this report -
The France Pharmacovigilance Market Size is anticipated to reach USD 636.02 Million by 2035, growing at a CAGR of 7.47% from 2025 to 2035. The France pharmacovigilance market is driven by stringent regulatory frameworks, increasing adverse drug reaction (ADR) reporting, and growing adoption of pharmacovigilance software by pharmaceutical companies.
Market Overview
Pharmacovigilance is the science and practice of monitoring the safety of medicines, aimed at identifying and evaluating adverse drug reactions (ADRs) and ensuring patient safety. In France, the pharmacovigilance market is structured around a comprehensive national system coordinated by the French National Agency for Medicines and Health Products Safety (ANSM). The country operates a network of regional pharmacovigilance centers that collect and analyze drug safety data. France’s pharmacovigilance framework is closely aligned with the European Medicines Agency (EMA), enabling efficient data sharing and harmonization of safety protocols across the EU. The market encompasses a wide range of services, including ADR reporting, risk management, post-marketing surveillance, and regulatory compliance. Pharmaceutical companies, healthcare providers, and government bodies work collaboratively to maintain drug safety standards. With a strong emphasis on transparency, data accuracy, and compliance, the French pharmacovigilance system is considered one of the most robust in Europe, contributing to the overall health ecosystem.
Report Coverage
This research report categorizes the market for the France pharmacovigilance market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the France pharmacovigilance market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the France pharmacovigilance market.
France Pharmacovigilance Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 288.09 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 7.47% |
| 2035 Value Projection: | USD 636.02 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 230 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Product Life Cycle (Pre-clinical, Phase I, Phase II, Phase III, and Phase IV), By Service Provider (In-house and Contract Outsourcing) |
| Companies covered:: | Sanofi, Servier, Ipsen, Laboratoires Expanscience, AB Cube, Parexel, Capgemini, Bayer AG, Arithmos, and Others |
| Pitfalls & Challenges: | COVID-19 Impact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Strict regulatory oversight, increased reporting of adverse drug reactions (ADRs), and heightened public awareness of medication safety are key factors propelling the pharmacovigilance sector in France. Expansion in clinical trials and growing pharmaceutical consumption demand more rigorous drug monitoring systems. Technological advancements, including AI and data analytics, are streamlining signal detection and improving response times. Additionally, integration with European Union safety standards encourages pharmaceutical companies to adopt comprehensive pharmacovigilance solutions to maintain compliance and protect public health.
Restraining Factors
Limited awareness among patients, underreporting of adverse drug reactions, and high costs associated with implementing advanced pharmacovigilance systems are key restraining factors. Additionally, data privacy concerns and complexities in regulatory compliance across regions can hinder efficient pharmacovigilance operations, affecting the overall growth of the market in France.
Market Segmentation
The France pharmacovigilance market share is classified into product life cycle and service provider.
- The phase IV segment held the highest market share in 2024 and is expected to grow at a remarkable CAGR during the forecast period.
The France pharmacovigilance market is segmented by product life cycle into pre-clinical, phase I, phase II, phase III, and phase IV. Among these, the phase IV segment held the highest market share in 2024 and is expected to grow at a remarkable CAGR during the forecast period. The growth of this segment is primarily driven by the increasing need for long-term safety data, the emergence of rare or delayed adverse effects, and stricter regulatory requirements for post-marketing safety monitoring. Pharmaceutical companies are placing greater emphasis on phase IV studies to ensure continued compliance and to maintain public trust in their products.
- The contract outsourcing segment held the largest market share in 2024 and is expected to grow at a substantial CAGR during the forecast period.
The France pharmacovigilance market is segmented by service provider into in-house and contract outsourcing. Among these, the contract outsourcing segment held the largest market share in 2024 and is expected to grow at a substantial CAGR during the forecast period. The growth can be attributed to the increasing demand for specialized expertise, cost efficiency, and the need for scalable solutions in pharmacovigilance activities. Many pharmaceutical companies are opting to outsource pharmacovigilance services to third-party vendors, enabling them to focus on core operations while ensuring compliance with regulatory requirements.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the France pharmacovigilance market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Sanofi
- Servier
- Ipsen
- Laboratoires Expanscience
- AB Cube
- Parexel
- Capgemini
- Bayer AG
- Arithmos
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at France, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the France pharmacovigilance market based on the below-mentioned segments:
France Pharmacovigilance Market, By Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
France Pharmacovigilance Market, By Service Provider
- In-house
- Contract Outsourcing
Need help to buy this report?